Stem cell-derived tissue-associated regulatory T cells ameliorate the development of autoimmunity
- PMID: 26846186
- PMCID: PMC4742827
- DOI: 10.1038/srep20588
Stem cell-derived tissue-associated regulatory T cells ameliorate the development of autoimmunity
Abstract
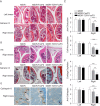
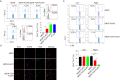
Pluripotent stem cells (PSCs) have the potential to produce almost all of the cells in the body, including regulatory T cells (Tregs). However, the exact conditions required for the development of antigen (Ag)-specific Tregs from PSCs (i.e., PSC-Tregs) are not well delineated. Ag-specific PSC-Tregs can be tissue/organ-associated and migrate to local inflamed tissues/organs to suppress the autoimmune response after adoptive transfer, thereby avoiding potential overall immunosuppression from non-specific Tregs. In this study, we developed a new approach to generate functional Ag-specific Tregs from induced PSCs (iPSCs), i.e., iPSC-Tregs, which had the ability to generate an Ag-specific immunosuppressive response in a murine model of arthritis. We retrovirally transduced murine iPSCs with a construct containing genes of Ag-specific T cell receptor (TCR) and the transcriptional factor FoxP3. We differentiated the iPSCs into Ag-specific iPSC-Tregs using in vitro or in vivo Notch signaling, and demonstrated that adoptive transfer of such Tregs dramatically suppressed autoimmunity in a well-established Ag-induced arthritis model, including the inflammation, joint destruction, cartilage prostaglandin depletion, osteoclast activity, and Th17 production. Our results indicate that PSCs can be used to develop Ag-specific Tregs, which have a therapeutic potential for Treg-based therapies of autoimmune disorders.
Figures




Similar articles
-
Stem cell-derived tissue-associated regulatory T cells suppress the activity of pathogenic cells in autoimmune diabetes.JCI Insight. 2019 Apr 4;4(7):e126471. doi: 10.1172/jci.insight.126471. eCollection 2019 Apr 4. JCI Insight. 2019. PMID: 30777937 Free PMC article.
-
Development of Stem Cell-derived Antigen-specific Regulatory T Cells Against Autoimmunity.J Vis Exp. 2016 Nov 8;(117):54720. doi: 10.3791/54720. J Vis Exp. 2016. PMID: 27911371 Free PMC article.
-
Adoptive transfer of all-trans-retinal-induced regulatory T cells ameliorates experimental autoimmune arthritis in an interferon-gamma knockout model.Autoimmunity. 2012 Sep;45(6):460-9. doi: 10.3109/08916934.2012.682666. Epub 2012 Jun 14. Autoimmunity. 2012. PMID: 22559266
-
Targeting Stem Cell-Derived Tissue-Associated Regulatory T Cells for Type 1 Diabetes Immunotherapy.Curr Diab Rep. 2019 Aug 30;19(10):89. doi: 10.1007/s11892-019-1213-7. Curr Diab Rep. 2019. PMID: 31471667 Free PMC article. Review.
-
Development of Auto Antigen-specific Regulatory T Cells for Diabetes Immunotherapy.Immune Netw. 2016 Oct;16(5):281-285. doi: 10.4110/in.2016.16.5.281. Epub 2016 Oct 25. Immune Netw. 2016. PMID: 27799873 Free PMC article. Review.
Cited by
-
Stem Cell-Derived Viral Antigen-Specific T Cells Suppress HIV Replication and PD-1 Expression on CD4+ T Cells.Viruses. 2021 Apr 25;13(5):753. doi: 10.3390/v13050753. Viruses. 2021. PMID: 33923025 Free PMC article.
-
Regulatory T Cell Therapy of Graft-versus-Host Disease: Advances and Challenges.Int J Mol Sci. 2021 Sep 7;22(18):9676. doi: 10.3390/ijms22189676. Int J Mol Sci. 2021. PMID: 34575843 Free PMC article. Review.
-
Stem cell therapy for abrogating stroke-induced neuroinflammation and relevant secondary cell death mechanisms.Prog Neurobiol. 2017 Nov;158:94-131. doi: 10.1016/j.pneurobio.2017.07.004. Epub 2017 Jul 23. Prog Neurobiol. 2017. PMID: 28743464 Free PMC article. Review.
-
Regulatory T cells in rheumatoid arthritis: functions, development, regulation, and therapeutic potential.Cell Mol Life Sci. 2022 Sep 29;79(10):533. doi: 10.1007/s00018-022-04563-0. Cell Mol Life Sci. 2022. PMID: 36173485 Free PMC article. Review.
-
NAC1 modulates autoimmunity by suppressing regulatory T cell-mediated tolerance.Sci Adv. 2022 Jul;8(26):eabo0183. doi: 10.1126/sciadv.abo0183. Epub 2022 Jun 29. Sci Adv. 2022. PMID: 35767626 Free PMC article.
References
-
- Wing J. B., Ise W., Kurosaki T. & Sakaguchi S. Regulatory T Cells Control Antigen-Specific Expansion of Tfh Cell Number and Humoral Immune Responses via the Coreceptor CTLA-4. Immunity 41, 1013–1025 (2014). - PubMed
-
- Maeda Y. et al. Detection of self-reactive CD8(+) T cells with an anergic phenotype in healthy individuals. Science 346, 1536–1540 (2014). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

