Overexpression of uncoupling protein 2 inhibits the high glucose-induced apoptosis of human umbilical vein endothelial cells
- PMID: 26846204
- PMCID: PMC4771113
- DOI: 10.3892/ijmm.2016.2478
Overexpression of uncoupling protein 2 inhibits the high glucose-induced apoptosis of human umbilical vein endothelial cells
Abstract
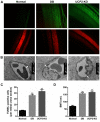
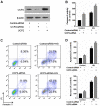
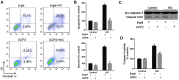
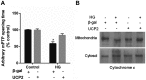
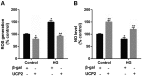
Ectopic apoptosis of vascular cells plays a critical role in the early stage development of diabetic retinopathy (DR). Uncoupling protein 2 (UCP2) is a mitochondrial modulator which protects against endothelial dysfunction. However, the role which UCP2 plays in endothelial apoptosis and its association with DR was unclear. In the present study, we investigated whether UCP2 functioned as an inhibitor of DR in endothelial cells. Firstly, we noted that in UCP2‑knockout mice retinal cell death and damage in vivo was similar to that of db/db diabetic mice. Additionally, UCP2 knockdown induced caspase-3 activation and exaggerated high glucose (HG)-induced apoptosis of human umbilical vein endothelial cells (HUVECs). Conversely, adenovirus-mediated UCP2 overexpression inhibited the apoptosis of HUVECs and HG-induced caspase-3 activation. Furthermore, HG treatment resulted in the opening of the permeability transition pore (PTP) and liberation of cytochrome c from mitochondria to the cytosol in HUVECs. Notably, UCP2 overexpression inhibited these processes. Furthermore, adenovirus-mediated UCP2 overexpression led to a significant increase in intracellular nitric oxide (NO) levels and a decrease in reactive oxygen species (ROS) generation in HUVECs. Collectively, these data suggest that UCP2 plays an anti-apoptotic role in endothelial cells. Thus, we suggest that approaches which augment UCP2 expression in vascular endothelial cells aid in preventing the early stage development and progression of DR.
Figures

References
-
- Cerani A, Tetreault N, Menard C, Lapalme E, Patel C, Sitaras N, Beaun F, Leboeuf D, De Guire V, Binet F, et al. Neuron-derived semaphorin 3A is an early inducer of vascular permeability in diabetic retinopathy via neuropilin-1. Cell Metab. 2013;18:505–518. doi: 10.1016/j.cmet.2013.09.003. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

