Tetradecylthiopropionic acid induces hepatic mitochondrial dysfunction and steatosis, accompanied by increased plasma homocysteine in mice
- PMID: 26846427
- PMCID: PMC4743328
- DOI: 10.1186/s12944-016-0192-9
Tetradecylthiopropionic acid induces hepatic mitochondrial dysfunction and steatosis, accompanied by increased plasma homocysteine in mice
Abstract
Background: Hepatic mitochondrial dysfunction plays an important role in the pathogenesis of non-alcoholic fatty liver disease (NAFLD). Methyl donor supplementation has been shown to alleviate NAFLD, connecting the condition to the one-carbon metabolism. Thus, the objective was to investigate regulation of homocysteine (Hcy) and metabolites along the choline oxidation pathway during induction of hepatic steatosis by the fatty acid analogue tetradecylthiopropionic acid (TTP), an inhibitor of mitochondrial fatty acid oxidation.
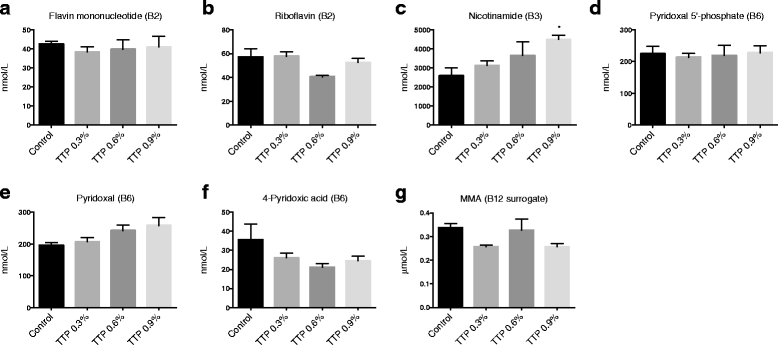
Methods: Mice were fed a control diet, or diets containing 0.3 %, 0.6 %, or 0.9 % (w/w) TTP for 14 days. Blood and liver samples were collected, enzyme activities and gene expression were analyzed in liver, lipid and fatty acid composition in liver and plasma, one-carbon metabolites, B-vitamin status, carnitine and acylcarnitines were analyzed in plasma.
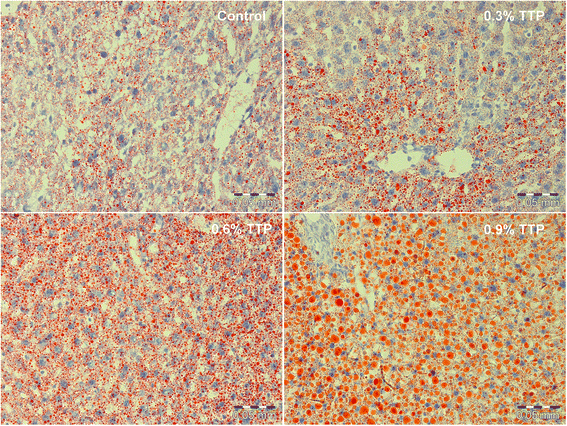
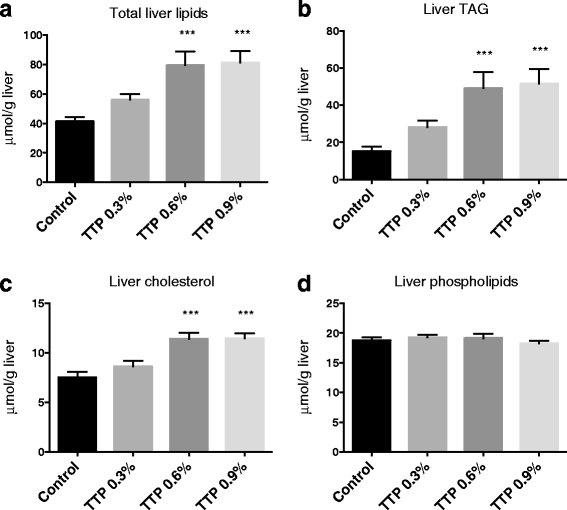
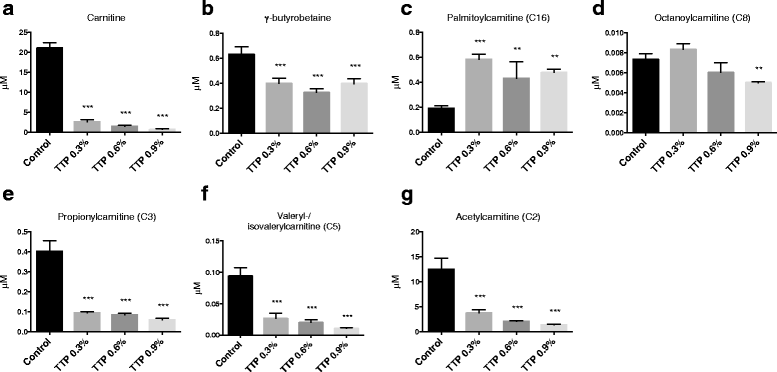
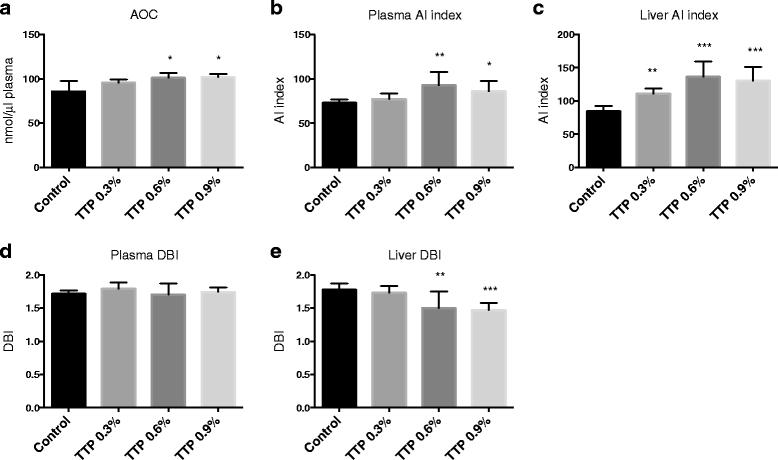
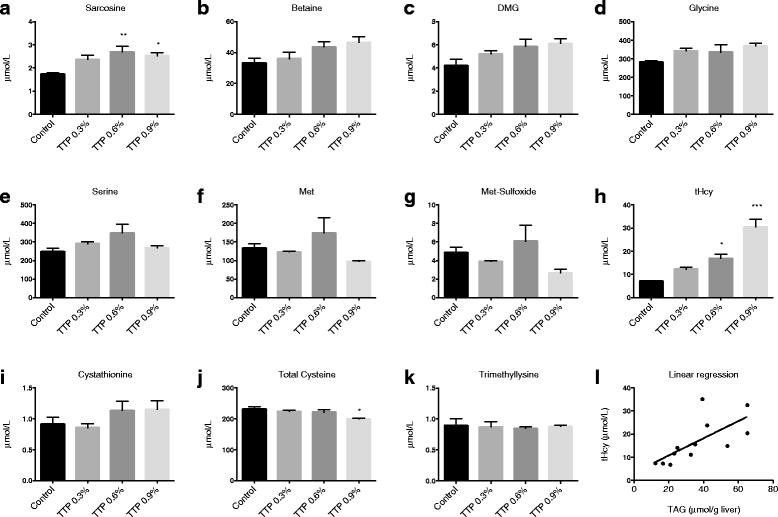
Results: Liver mitochondrial fatty acid oxidation decreased by 40 % and steatosis was induced in a dose dependent manner; total lipids increased 1.6-fold in animals treated with 0.3 % TTP, 2-fold with 0.6 % TTP and 2.1 fold with 0.9 % TTP compared to control. The higher hepatic concentration of fatty acids was associated with shortening of carbon-length. Furthermore, the inhibited fatty acid oxidation led to a 30-fold decrease in plasma carnitine and 9.3-fold decrease in acetylcarnitine at the highest dose of TTP, whereas an accumulation of palmitoylcarnitine resulted. Compared to the control diet, TTP administration was associated with elevated plasma total Hcy (control: 7.2 ± 0.3 umol/L, 0.9 % TTP: 30.5 ± 5.9 umol/L) and 1.4-1.6 fold increase in the one-carbon metabolites betaine, dimethylglycine, sarcosine and glycine, accompanied by changes in gene expression of the different B-vitamin dependent pathways of Hcy and choline metabolism. A positive correlation between total Hcy and hepatic triacylglycerol resulted.
Conclusions: The TTP-induced inhibition of mitochondrial fatty acid oxidation was not associated with increased hepatic oxidative stress or inflammation. Our data suggest a link between mitochondrial dysfunction and the methylation processes within the one-carbon metabolism in mice.
Figures


Similar articles
-
Hepatic steatosis induced in C57BL/6 mice by a non-ß oxidizable fatty acid analogue is associated with reduced plasma kynurenine metabolites and a modified hepatic NAD+/NADH ratio.Lipids Health Dis. 2020 May 14;19(1):94. doi: 10.1186/s12944-020-01271-1. Lipids Health Dis. 2020. PMID: 32410680 Free PMC article.
-
Associations between fatty acid oxidation, hepatic mitochondrial function, and plasma acylcarnitine levels in mice.Nutr Metab (Lond). 2018 Jan 29;15:10. doi: 10.1186/s12986-018-0241-7. eCollection 2018. Nutr Metab (Lond). 2018. PMID: 29422939 Free PMC article.
-
Hepatic fatty acid metabolism as a determinant of plasma and liver triacylglycerol levels. Studies on tetradecylthioacetic and tetradecylthiopropionic acids.Eur J Biochem. 1995 Feb 1;227(3):715-22. doi: 10.1111/j.1432-1033.1995.tb20193.x. Eur J Biochem. 1995. PMID: 7867630
-
Mitochondrial dysfunction in non-alcoholic fatty liver disease and insulin resistance: cause or consequence?Free Radic Res. 2013 Nov;47(11):854-68. doi: 10.3109/10715762.2013.830717. Epub 2013 Oct 4. Free Radic Res. 2013. PMID: 23915028 Review.
-
Nonalcoholic Fatty liver disease: pathogenesis and therapeutics from a mitochondria-centric perspective.Oxid Med Cell Longev. 2014;2014:637027. doi: 10.1155/2014/637027. Epub 2014 Oct 13. Oxid Med Cell Longev. 2014. PMID: 25371775 Free PMC article. Review.
Cited by
-
The Association between Non-Alcoholic Fatty Liver Disease (NAFLD) and Advanced Fibrosis with Serological Vitamin B12 Markers: Results from the NHANES 1999-2004.Nutrients. 2022 Mar 14;14(6):1224. doi: 10.3390/nu14061224. Nutrients. 2022. PMID: 35334881 Free PMC article.
-
Mitochondrial Dysfunction in Metabolic Dysfunction Fatty Liver Disease (MAFLD).Int J Mol Sci. 2023 Dec 15;24(24):17514. doi: 10.3390/ijms242417514. Int J Mol Sci. 2023. PMID: 38139341 Free PMC article. Review.
-
Maternal high-fat diet disrupted one-carbon metabolism in offspring, contributing to nonalcoholic fatty liver disease.Liver Int. 2021 Jun;41(6):1305-1319. doi: 10.1111/liv.14811. Epub 2021 Feb 16. Liver Int. 2021. PMID: 33529448 Free PMC article.
-
Hepatic steatosis induced in C57BL/6 mice by a non-ß oxidizable fatty acid analogue is associated with reduced plasma kynurenine metabolites and a modified hepatic NAD+/NADH ratio.Lipids Health Dis. 2020 May 14;19(1):94. doi: 10.1186/s12944-020-01271-1. Lipids Health Dis. 2020. PMID: 32410680 Free PMC article.
-
Associations between fatty acid oxidation, hepatic mitochondrial function, and plasma acylcarnitine levels in mice.Nutr Metab (Lond). 2018 Jan 29;15:10. doi: 10.1186/s12986-018-0241-7. eCollection 2018. Nutr Metab (Lond). 2018. PMID: 29422939 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

