Advances in understanding erythropoiesis: evolving perspectives
- PMID: 26846448
- PMCID: PMC4833665
- DOI: 10.1111/bjh.13938
Advances in understanding erythropoiesis: evolving perspectives
Abstract
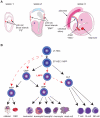
Red blood cells (RBCs) are generated from haematopoietic stem and progenitor cells (HSPCs) through the step-wise process of differentiation known as erythropoiesis. In this review, we discuss our current understanding of erythropoiesis and highlight recent advances in this field. During embryonic development, erythropoiesis occurs in three distinct waves comprising first, the yolk sac-derived primitive RBCs, followed sequentially by the erythro-myeloid progenitor (EMP) and HSPC-derived definitive RBCs. Recent work has highlighted the complexity and variability that may exist in the hierarchical arrangement of progenitors responsible for erythropoiesis. Using recently defined cell surface markers, it is now possible to enrich for erythroid progenitors and precursors to a much greater extent than has been possible before. While a great deal of knowledge has been gained on erythropoiesis from model organisms, our understanding of this process is currently being refined through human genetic studies. Genes mutated in erythroid disorders can now be identified more rapidly by the use of next-generation sequencing techniques. Genome-wide association studies on erythroid traits in healthy populations have also revealed new modulators of erythropoiesis. All of these recent developments have significant promise not only for increasing our understanding of erythropoiesis, but also for improving our ability to intervene when RBC production is perturbed in disease.
Keywords: erythropoiesis; haematopoiesis; haemopoietic progenitors; red cell disorders; red cells.
© 2016 John Wiley & Sons Ltd.
Figures



References
-
- Arlet JB, Ribeil JA, Guillem F, Negre O, Hazoume A, Marcion G, Beuzard Y, Dussiot M, Moura IC, Demarest S, de Beauchene IC, Belaid-Choucair Z, Sevin M, Maciel TT, Auclair C, Leboulch P, Chretien S, Tchertanov L, Baudin-Creuza V, Seigneuric R, Fontenay M, Garrido C, Hermine O, Courtois G. HSP70 sequestration by free alpha-globin promotes ineffective erythropoiesis in beta-thalassaemia. Nature. 2014;514:242–246. - PubMed
-
- Basak A, Hancarova M, Ulirsch JC, Balci TB, Trkova M, Pelisek M, Vlckova M, Muzikova K, Cermak J, Trka J, Dyment DA, Orkin SH, Daly MJ, Sedlacek Z, Sankaran VG. BCL11A deletions result in fetal hemoglobin persistence and neurodevelopmental alterations. Journal of Clinical Investigation. 2015;125:2363–2368. - PMC - PubMed
-
- Bauer DE, Kamran SC, Lessard S, Xu J, Fujiwara Y, Lin C, Shao Z, Canver MC, Smith EC, Pinello L, Sabo PJ, Vierstra J, Voit RA, Yuan GC, Porteus MH, Stamatoyannopoulos JA, Lettre G, Orkin SH. An erythroid enhancer of BCL11A subject to genetic variation determines fetal hemoglobin level. Science. 2013;342:253–257. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

