Multicentric randomized clinical trial to evaluate the long-term effectiveness of a motivational intervention against smoking, based on the information obtained from spirometry in primary care: the RESET study protocol
- PMID: 26846522
- PMCID: PMC4743363
- DOI: 10.1186/s12875-016-0415-1
Multicentric randomized clinical trial to evaluate the long-term effectiveness of a motivational intervention against smoking, based on the information obtained from spirometry in primary care: the RESET study protocol
Abstract
Background: Spirometry is the recommended method of evaluating pulmonary function when respiratory disease is suspected in smokers. Nonetheless, no evidence exists of the usefulness of information obtained from this test as a motivational strategy for smoking cessation. The primary objective of this study is to evaluate the effectiveness of a motivational intervention based on spirometry results in achieving long-term smoking cessation.
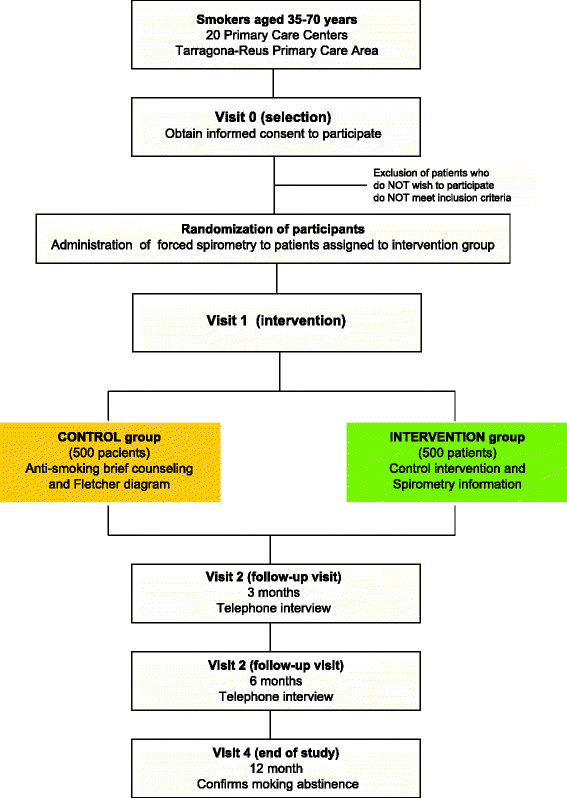
Methods/design: We propose a multicenter randomized clinical trial in the primary care setting.
Study subjects: We will recruit active smokers of both sexes, aged 35-70 years, with a cumulated smoking habit exceeding 10 packs/year and who consult for any reason with their primary care physician in the 20 health centers in the province of Tarragona (Spain). Patients with a history of lung disease or who have undergone exploratory measures of pulmonary function in the preceding 12 months will be excluded. All patients who agree to participate will provide signed informed consent prior to their inclusion. A total of 1000 smokers will be consecutively randomized to a control or intervention group (1:1).
Intervention: Participants in both groups will receive brief (5-minute) health counseling, in accordance with usual clinical practice. In a consultation lasting about 15 minutes, participants in the intervention group will also receive detailed, personalized information about the results of a spirometry test and about their lung age compared with their chronological age. Both groups will be followed up for 12 months. Main variables and analysis: The main variable will be sustained smoking abstinence at 12 months after the intervention, as confirmed by CO breath testing and urine cotinine test. Results will be analyzed based on intention to treat, using the chi-square test and logistical regression if necessary to adjust for confounding variables.
Discussion: We expect the rate of prolonged smoking abstinence in the intervention group will be at least 5% higher than in the control group. If this strategy proves effective, it could easily be included in the health promotion activities offered in primary care settings.
Trial registration: ClinicalTrials.gov Identifier: NCT02153047 . Registered on 28/05/2014.
Figures
References
-
- Doll R, Peto R, Boreham J, Sutherland I. Mortality in relation to smoking: 50 years’ observations on male British doctors. BMJ. 2004;328:1519–1533. doi: 10.1136/bmj.38142.554479.AE. - DOI - PMC - PubMed
-
- Ministerio de Sanidad y Consumo. Plan de Calidad para el sistema Nacional de Salud. Estrategia en EPOC del Sistema Nacional de Salud. Sanidad 2009. Ministerio de Sanidad y Política Social. Available at: http://www.msc.es/organizacion/sns/planCalidadSNS/docs/EstrategiaEPOCSNS....
-
- AHRQ. Agency for Healthcare Research and Quality (US) The Guide to Clinical Preventive Services 2014: Recommendations of the U.S. Preventive Services Task Force. Rockville: Agency for Healthcare Research and Quality (US); 2014. - PubMed
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical