Knowledge about Human Papillomavirus and Time to Complete Vaccination among Vulnerable Female Youth
- PMID: 26846571
- PMCID: PMC4808615
- DOI: 10.1016/j.jpeds.2015.12.070
Knowledge about Human Papillomavirus and Time to Complete Vaccination among Vulnerable Female Youth
Abstract
Objective: To examine the association of knowledge about human papillomavirus (HPV) on the time to completion of the 3-dose quadrivalent vaccine series in an inner-city population of adolescent female subjects at high risk for infection.
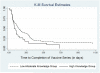
Study design: We prospectively followed 139 female subjects aged 14-20 years enrolled in a vaccine surveillance study in New York City during a period of at least 24 months. Participants were given a 30-item true or false survey on HPV at enrollment and ranked according to the number of correct responses. Multivariate Cox regression was used to examine the association between level of knowledge about HPV and time to completion (in days) of vaccine dose 1-3, dose 1-2, and dose 2-3.
Results: Overall time to completion of the 3-dose vaccine ranged from 158 days to 1114 days. Participants in the high knowledge group (top quartile) were significantly more likely to complete the 3-dose series earlier (hazard ratio 1.69, 95% CI 1.03-2.77; P = .04), in particular doses 2-3 (hazard ratio 1.71, 95% CI 1.02-2.89; P = .04), than those with low-to-moderate knowledge (bottom 3 quartiles).
Conclusions: These findings suggest that knowledge of HPV is associated with shorter time to complete the 3-dose HPV vaccine series. Educational campaigns at time of vaccination may be important to improve vaccine adherence.
Copyright © 2016 Elsevier Inc. All rights reserved.
Figures
References
-
- Quadrivalent Vaccine against Human Papillomavirus to Prevent High-Grade Cervical Lesions. New England Journal of Medicine. 2007;356:1915–1927. - PubMed
-
- Garland S, Hernandez-Avila M, Wheeler C, Perez G, Harper D, Leodolter S, et al. Quadrivalent Vaccine against Human Papillomavirus to Prevent Anogenital Diseases. New England Journal of Medicine. 2007;356:1928–1943. - PubMed
-
- Markowitz LE, Dunne EF, Saraiya M, Chesson H, Curtis CR, Gee J, et al. Human papillomavirus vaccine: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morbidity and Mortality Weekly Recommendations. 2014;63:1–23. - PubMed
-
- World Health Organization Meeting of the Strategic Advisory Group of Experts on immunization, April 2014 – conclusions and recommendations. Wkly Epidemiol Rec. 2014;89:221–36. 2015. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources