Three-times-weekly, post-dialysis cefepime therapy in patients on maintenance hemodialysis: a retrospective study
- PMID: 26846675
- PMCID: PMC4743204
- DOI: 10.1186/s40360-016-0048-y
Three-times-weekly, post-dialysis cefepime therapy in patients on maintenance hemodialysis: a retrospective study
Abstract
Background: In hemodialysis patients, post-dialysis treatment with intravenous antibiotics permits even severe infections to be managed on an outpatient basis. Cefepime is a fourth-generation cephalosporin with a broad spectrum of action in monotherapy. We report on the pharmacokinetics of cefepime in post-dialysis therapy.
Methods: Since June 2012, twelve infections were treated with post-dialysis cefepime in 9 patients on high-flux hemodialysis. The initial post-dialysis dose of cefepime was approximately 15 mg/kg. The following doses were adapted according to the trough serum levels obtained before the subsequent dialysis in order to be above the EUCAST breakpoints for susceptible organisms and above the MIC90. Residual plasma concentrations were determined before (n = 30) and after (n = 17) dialysis by liquid chromatography-mass spectrometry.
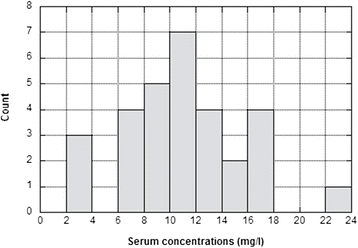
Results: Overall, the mean ± SD dose of cefepime was 920 ± 270 mg (14.5 ± 5.1 mg/kg), but it was significantly lower before the 48 h interval (775 ± 210 mg or 12.7 ± 4.5 mg/kg) compared to the 72 h interval (1125 ± 225 mg or 17.2 ± 4.9 mg/kg) (p < 0.05). The mean trough pre-dialysis concentrations were 10.7 ± 3.9 mg/l and 11.3 ± 5.6 mg/l at 48 and 72 h, respectively. These levels always largely exceeded the EUCAST susceptibility breakpoints for all the targeted bacteria (>1 mg/l) with the exception of Pseudomonas aeruginosa (>8 mg/l). Cefepime concentrations were higher in anuric patients compared to those with preserved diuresis (15.6 ± 3.5 vs 9.25 ± 3.6 mg/l; p < 0.001) and decreased on average by 81 % during dialysis (from 10.5 ± 3.7 to 1.96 ± 1.2 mg/l; p < 0.001). The clinical outcome of all patients was good.
Conclusions: Outpatient treatment with cefepime administered post-dialysis three-times-weekly was effective and well-tolerated in our patients. According to our data, in patients infected by highly susceptible pathogens a fixed dose of cefepime of 1 g before every 48-h interval and of 1.5 g before every 72-h interval should be recommended, without need of routine monitoring of the cefepime blood levels. In patients having an infection with less susceptibles pathogens as P. aeruginosa, and particularly in those among them exhibiting residual renal function, higher initial doses are necessary (1.5 g before a 48-h interval and 2.0 g before a 72-h interval) with adaption according to the subsequent pre-dialysis trough serum levels.
Figures
Similar articles
-
Pharmacokinetic-pharmacodynamic rationale for cefepime dosing regimens in intensive care units.J Antimicrob Chemother. 2006 Nov;58(5):987-93. doi: 10.1093/jac/dkl349. Epub 2006 Aug 30. J Antimicrob Chemother. 2006. PMID: 16943209
-
Cefepime in critically ill patients: continuous infusion vs. an intermittent dosing regimen.Int J Clin Pharmacol Ther. 2005 Aug;43(8):360-9. doi: 10.5414/cpp43360. Int J Clin Pharmacol Ther. 2005. PMID: 16119511 Clinical Trial.
-
Comparison of the prevalence of convulsions associated with the use of cefepime and meropenem.Int J Clin Pharm. 2013 Oct;35(5):683-7. doi: 10.1007/s11096-013-9799-3. Epub 2013 Jun 4. Int J Clin Pharm. 2013. PMID: 23733559
-
Cefiderocol: A Siderophore Cephalosporin with Activity Against Carbapenem-Resistant and Multidrug-Resistant Gram-Negative Bacilli.Drugs. 2019 Feb;79(3):271-289. doi: 10.1007/s40265-019-1055-2. Drugs. 2019. PMID: 30712199 Review.
-
In-vitro profile of a new beta-lactam, ceftobiprole, with activity against methicillin-resistant Staphylococcus aureus.Clin Microbiol Infect. 2007 Jun;13 Suppl 2:17-24. doi: 10.1111/j.1469-0691.2007.01722.x. Clin Microbiol Infect. 2007. PMID: 17488372 Review.
Cited by
-
Cefepime Neurotoxicity in Acute Kidney Injury: The Importance of Renal Dosing.Case Rep Nephrol. 2025 Apr 11;2025:2274647. doi: 10.1155/crin/2274647. eCollection 2025. Case Rep Nephrol. 2025. PMID: 40256197 Free PMC article.
-
Post-Dialysis Parenteral Antimicrobial Therapy in Patients Receiving Intermittent High-Flux Hemodialysis.Drugs. 2021 Apr;81(5):555-574. doi: 10.1007/s40265-021-01469-2. Epub 2021 Feb 16. Drugs. 2021. PMID: 33591549 Free PMC article. Review.
-
Comment on: "Post-Dialysis Parenteral Antimicrobial Therapy in Patients Receiving Intermittent High-Flux Haemodialysis".Drugs. 2021 Jun;81(9):1121-1123. doi: 10.1007/s40265-021-01535-9. Epub 2021 May 26. Drugs. 2021. PMID: 34037962 No abstract available.
-
Cefepime-Induced Encephalopathy With Seizures in a Pediatric Patient With End-Stage Renal Disease Rapidly Reversed by High-Efficiency Hemodialysis.Cureus. 2021 Mar 12;13(3):e13842. doi: 10.7759/cureus.13842. Cureus. 2021. PMID: 33717772 Free PMC article.
-
Removal of common antimicrobial agents by sustained low-efficiency dialysis.Antimicrob Agents Chemother. 2024 Mar 6;68(3):e0157923. doi: 10.1128/aac.01579-23. Epub 2024 Feb 13. Antimicrob Agents Chemother. 2024. PMID: 38349160 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical