BKM-120 (Buparlisib): A Phosphatidyl-Inositol-3 Kinase Inhibitor with Anti-Invasive Properties in Glioblastoma
- PMID: 26846842
- PMCID: PMC4742861
- DOI: 10.1038/srep20189
BKM-120 (Buparlisib): A Phosphatidyl-Inositol-3 Kinase Inhibitor with Anti-Invasive Properties in Glioblastoma
Abstract
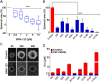
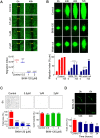
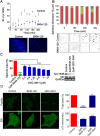
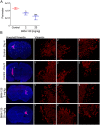
Glioblastoma is an aggressive, invasive tumor of the central nervous system (CNS). There is a widely acknowledged need for anti-invasive therapeutics to limit glioblastoma invasion. BKM-120 is a CNS-penetrant pan-class I phosphatidyl-inositol-3 kinase (PI3K) inhibitor in clinical trials for solid tumors, including glioblastoma. We observed that BKM-120 has potent anti-invasive effects in glioblastoma cell lines and patient-derived glioma cells in vitro. These anti-migratory effects were clearly distinguishable from cytostatic and cytotoxic effects at higher drug concentrations and longer durations of drug exposure. The effects were reversible and accompanied by changes in cell morphology and pronounced reduction in both cell/cell and cell/substrate adhesion. In vivo studies showed that a short period of treatment with BKM-120 slowed tumor spread in an intracranial xenografts. GDC-0941, a similar potent and selective PI3K inhibitor, only caused a moderate reduction in glioblastoma cell migration. The effects of BKM-120 and GDC-0941 were indistinguishable by in vitro kinase selectivity screening and phospho-protein arrays. BKM-120 reduced the numbers of focal adhesions and the velocity of microtubule treadmilling compared with GDC-0941, suggesting that mechanisms in addition to PI3K inhibition contribute to the anti-invasive effects of BKM-120. Our data suggest the CNS-penetrant PI3K inhibitor BKM-120 may have anti-invasive properties in glioblastoma.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

