PPARα (Peroxisome Proliferator-activated Receptor α) Activation Reduces Hepatic CEACAM1 Protein Expression to Regulate Fatty Acid Oxidation during Fasting-refeeding Transition
- PMID: 26846848
- PMCID: PMC4825014
- DOI: 10.1074/jbc.M116.714014
PPARα (Peroxisome Proliferator-activated Receptor α) Activation Reduces Hepatic CEACAM1 Protein Expression to Regulate Fatty Acid Oxidation during Fasting-refeeding Transition
Abstract
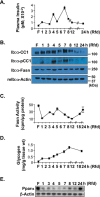
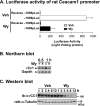
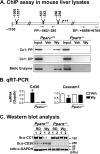
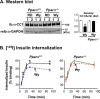
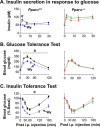
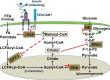
Carcinoembryonic antigen-related cell adhesion molecule 1 (CEACAM1) is expressed at high levels in the hepatocyte, consistent with its role in promoting insulin clearance in liver. CEACAM1 also mediates a negative acute effect of insulin on fatty acid synthase activity. Western blot analysis reveals lower hepatic CEACAM1 expression during fasting. Treating of rat hepatoma FAO cells with Wy14,643, an agonist of peroxisome proliferator-activated receptor α (PPARα), rapidly reduces Ceacam1 mRNA and CEACAM1 protein levels within 1 and 2 h, respectively. Luciferase reporter assay shows a decrease in the promoter activity of both rat and mouse genes by Pparα activation, and 5'-deletion and block substitution analyses reveal that the Pparα response element between nucleotides -557 and -543 is required for regulation of the mouse promoter activity. Chromatin immunoprecipitation analysis demonstrates binding of liganded Pparα toCeacam1promoter in liver lysates ofPparα(+/+), but notPparα(-/-)mice fed a Wy14,643-supplemented chow diet. Consequently, Wy14,643 feeding reduces hepatic Ceacam1 mRNA and CEACAM1 protein levels, thus decreasing insulin clearance to compensate for compromised insulin secretion and maintain glucose homeostasis and insulin sensitivity in wild-type mice. Together, the data show that the low hepatic CEACAM1 expression at fasting is mediated by Pparα-dependent mechanisms. Changes in CEACAM1 expression contribute to the coordination of fatty acid oxidation and insulin action in the fasting-refeeding transition.
Keywords: CEACAM1; PPAR response element; fatty acid metabolism; fatty acid synthase (FAS); gene expression; insulin clearance; insulin secretion; peroxisome proliferator-activated receptor (PPAR).
© 2016 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures

References
-
- Ader M., Stefanovski D., Kim S. P., Richey J. M., Ionut V., Catalano K. J., Hucking K., Ellmerer M., Van Citters G., Hsu I. R., Chiu J. D., Woolcott O. O., Harrison L. N., Zheng D., Lottati M., Kolka C. M., Mooradian V., Dittmann J., Yae S., Liu H., Castro A. V., Kabir M., and Bergman R. N. (2014) Hepatic insulin clearance is the primary determinant of insulin sensitivity in the normal dog. Obesity 22, 1238–1245 - PMC - PubMed
-
- Najjar S. M., Philippe N., Suzuki Y., Ignacio G. A., Formisano P., Accili D., and Taylor S. I. (1995) Insulin-stimulated phosphorylation of recombinant pp120/HA4, an endogenous substrate of the insulin receptor tyrosine kinase. Biochemistry 34, 9341–9349 - PubMed
-
- Formisano P., Najjar S. M., Gross C. N., Philippe N., Oriente F., Kern-Buell C. L., Accili D., and Gorden P. (1995) Receptor-mediated internalization of insulin: potential role of pp120/HA4, a substrate of the insulin receptor kinase. J. Biol. Chem. 270, 24073–24077 - PubMed
-
- Poy M. N., Yang Y., Rezaei K., Fernström M. A., Lee A. D., Kido Y., Erickson S. K., and Najjar S. M. (2002) CEACAM1 regulates insulin clearance in liver. Nat. Genet. 30, 270–276 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

