Anogenital distance as a marker of androgen exposure in humans
- PMID: 26846869
- PMCID: PMC6225986
- DOI: 10.1111/andr.12156
Anogenital distance as a marker of androgen exposure in humans
Abstract
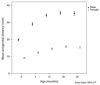
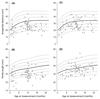
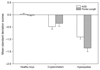
Abnormal foetal testis development has been proposed to underlie common disorders of the male reproductive system such as cryptorchidism, hypospadias, reduced semen quality and testicular germ cell tumour, which are regarded as components of a 'testicular dysgenesis syndrome'. The increasing trends and geographical variation in their incidence have been suggested to result from in utero exposure to environmental chemicals acting as endocrine disruptors. In rodents, the anogenital distance (AGD), measured from the anus to the base of genital tubercle, is a sensitive biomarker of androgen exposure during a critical embryonic window of testis development. In humans, several epidemiological studies have shown alterations in AGD associated with prenatal exposure to several chemicals with potential endocrine disrupting activity. However, the link between AGD and androgen exposure in humans is not well-defined. This review focuses on the current evidence for such a relationship. As in rodents, a clear gender difference is detected during foetal development of the AGD in humans which is maintained thereafter. Reduced AGD in association with clinically relevant outcomes of potential environmental exposures, such as cryptorchidism or hypospadias, is in keeping with AGD as a marker of foetal testicular function. Furthermore, AGD may reflect variations in prenatal androgen exposure in healthy children as shorter AGD at birth is associated with reduced masculine play behaviour in preschool boys. Several studies provide evidence linking shorter AGD with lower fertility, semen quality and testosterone levels in selected groups of adults attending andrology clinics. Overall, the observational data in humans are consistent with experimental studies in animals and support the use of AGD as a biomarker of foetal androgen exposure. Future studies evaluating AGD in relation to reproductive hormones in both infants and adults, and to gene polymorphisms, will help to further delineate the effect of prenatal and postnatal androgen exposures on AGD.
Keywords: anogenital distance; endocrine disrupters; masculanizing programming window; prenatal androgen exposure; testicular dysgenesis syndrome; testis development.
© 2016 American Society of Andrology and European Academy of Andrology.
Conflict of interest statement
Figures
References
-
- Abbott DH, Barnett DK, Bruns CM, Dumesic DA. Androgen excess fetal programming of female reproduction: a developmental aetiology for polycystic ovary syndrome? Hum Reprod Update. 2005;11:357–74. - PubMed
-
- Acerini CL, Miles HL, Dunger DB, Ong KK, Hughes IA. The descriptive epidemiology of congenital and acquired cryptorchidism in a UK infant cohort. Arch Dis Child. 2009;94:868–72. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials