The Sertoli cell: one hundred fifty years of beauty and plasticity
- PMID: 26846984
- PMCID: PMC5461925
- DOI: 10.1111/andr.12165
The Sertoli cell: one hundred fifty years of beauty and plasticity
Abstract
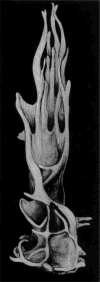
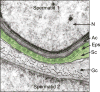
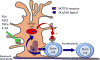
It has been one and a half centuries since Enrico Sertoli published the seminal discovery of the testicular 'nurse cell', not only a key cell in the testis, but indeed one of the most amazing cells in the vertebrate body. In this review, we begin by examining the three phases of morphological research that have occurred in the study of Sertoli cells, because microscopic anatomy was essentially the only scientific discipline available for about the first 75 years after the discovery. Biochemistry and molecular biology then changed all of biological sciences, including our understanding of the functions of Sertoli cells. Immunology and stem cell biology were not even topics of science in 1865, but they have now become major issues in our appreciation of Sertoli cell's role in spermatogenesis. We end with the universal importance and plasticity of function by comparing Sertoli cells in fish, amphibians, and mammals. In these various classes of vertebrates, Sertoli cells have quite different modes of proliferation and epithelial maintenance, cystic vs. tubular formation, yet accomplish essentially the same function but in strikingly different ways.
Keywords: Sertoli cell; amniotes; anamniotes; blood-testis barrier; germ cell; immune privilege; immune tolerance; spermatogenesis; stem cell niche; testis.
© 2016 American Society of Andrology and European Academy of Andrology.
Conflict of interest statement
The authors have no conflicts of interest to declare.
Figures


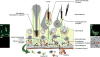


References
-
- Aflatoonian B, Ruban L, Jones M, Aflatoonian R, Fazeli A, Moore HD. In vitro post-meiotic germ cell development from human embryonic stem cells. Hum Reprod. 2009;24:3150–3159. - PubMed
-
- Almeida FF, Kristoffersen C, Taranger GL, Schulz RW. Spermatogenesis in Atlantic cod (Gadus morhua): a novel model of cystic germ cell development. Biol Reprod. 2008;78:27–34. - PubMed
-
- Andersson A-M. Inhibin B as a serum marker of spermatogenesis. In: Robaire B, Chemes H, Morales CR, editors. Andrology in the 21st Century. Medimond Publishing Company; New Jersey: 2001. pp. 123–130.
-
- Aponte PM, Soda T, Teerds KJ, Mizrak SC, van de Kant HJ, de Rooij DG. Propagation of bovine spermatogonial stem cells in vitro. Reproduction. 2008;136:543–557. - PubMed
Publication types
MeSH terms
Personal name as subject
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

