Breast Cancer MDA-MB-231 Cells Use Secreted Heat Shock Protein-90alpha (Hsp90α) to Survive a Hostile Hypoxic Environment
- PMID: 26846992
- PMCID: PMC4742873
- DOI: 10.1038/srep20605
Breast Cancer MDA-MB-231 Cells Use Secreted Heat Shock Protein-90alpha (Hsp90α) to Survive a Hostile Hypoxic Environment
Abstract
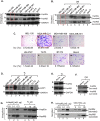
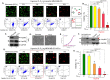
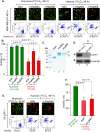
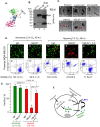
Rapidly growing tumours in vivo often outgrow their surrounding available blood supply, subjecting themselves to a severely hypoxic microenvironment. Understanding how tumour cells adapt themselves to survive hypoxia may help to develop new treatments of the tumours. Given the limited blood perfusion to the enlarging tumour, whatever factor(s) that allows the tumour cells to survive likely comes from the tumour cells themselves or its associated stromal cells. In this report, we show that HIF-1α-overexpressing breast cancer cells, MDA-MB-231, secrete heat shock protein-90alpha (Hsp90α) and use it to survive under hypoxia. Depletion of Hsp90α secretion from the tumour cells was permissive to cytotoxicity by hypoxia, whereas supplementation of Hsp90α-knockout tumour cells with recombinant Hsp90α, but not Hsp90β, protein prevented hypoxia-induced cell death via an autocrine mechanism through the LDL receptor-related protein-1 (LRP1) receptor. Finally, direct inhibition of the secreted Hsp90α with monoclonal antibody, 1G6-D7, enhanced tumour cell death under hypoxia. Therefore, secreted Hsp90α is a novel survival factor for certain tumours under hypoxia.
Figures
References
-
- Dales J. P. et al. Overexpression of hypoxia-inducible factor HIF-1alpha predicts early relapse in breast cancer: retrospective study in a series of 745 patients. Int. J. Cancer 116, 734–739 (2005). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

