Role of oral Minocycline in acute encephalitis syndrome in India - a randomized controlled trial
- PMID: 26847071
- PMCID: PMC4743094
- DOI: 10.1186/s12879-016-1385-6
Role of oral Minocycline in acute encephalitis syndrome in India - a randomized controlled trial
Abstract
Background: Acute encephalitis syndrome (AES) is a public health problem in India. Neuroinfections are believed to be the most important etiology. Minocycline is a semisythetic tetracycline having excellent penetration into cerebrospinal fluid, established neuroprotective and antiviral properties besides action on nonviral causes of AES. It has been shown to be effective in animal model of Japanese encephalitis (JE). A randomized, controlled trial of nasogastric/oral minocycline in JE and AES at a single centre in Uttar Pradesh, northern India, was therefore conducted.
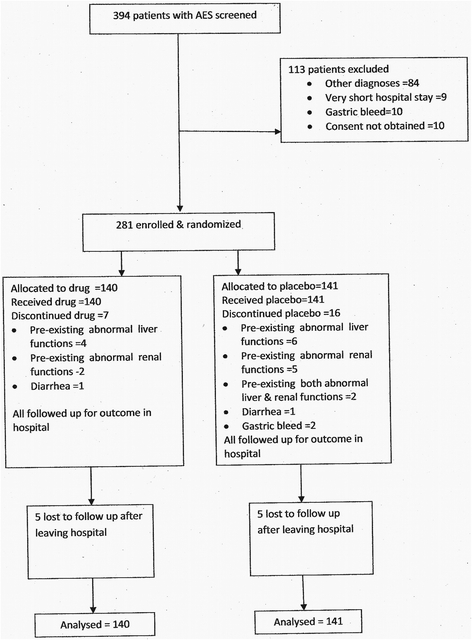
Methods: Patients beyond 3 years of age - but excluding women aged 16-44 years - hospitalized with AES of < =7 days duration were enrolled and block randomized to receive nasogastric/oral minocycline or placebo suspension and followed up. Patients, study personnel and those entering data were blinded as to drug or placebo received. Primary outcome was cumulative mortality at 3 months from hospitalization. Analysis was by intention to treat.
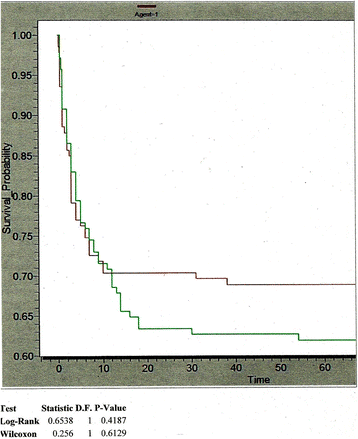
Results: 281 patients were enrolled, 140 received drug and 141 placebo. While there was no overall statistically significant difference in 3 month mortality between drug and placebo groups [RR = 0 · 83 (0 · 6-1 · 1)], there were encouraging trends in patients older than 12 years [RR = 0.70 (0.41-1.18)] and in Glasgow Outcome Score (GOS) at 3 months (χ(2) = 7 · 44, p = 0 · 059). These trends were further accentuated if patients dying within one day of reaching hospital were excluded [OR for 3 month mortality =0 · 70 (0 · 46-1 · 07), p = 0.090; 3 month GOS p = 0 · 028].
Conclusions: A trend towards better outcomes was observed with minocycline, especially in those patients who survived the initial day in hospital. These findings should form the basis for planning a larger study and possibly including minocycline in the initial management of AES as seen here.
Trial registration: The trial was registered with Clinical Trials Registry of India (CTRI) - CTRI/2010/091/006143.
Figures
Similar articles
-
Randomized, controlled trial of oral ribavirin for Japanese encephalitis in children in Uttar Pradesh, India.Clin Infect Dis. 2009 Feb 15;48(4):400-6. doi: 10.1086/596309. Clin Infect Dis. 2009. PMID: 19143532 Clinical Trial.
-
Trend of Japanese encephalitis in Uttar Pradesh, India from 2011 to 2013.Epidemiol Infect. 2016 Jan;144(2):363-70. doi: 10.1017/S0950268815000928. Epub 2015 Jun 26. Epidemiol Infect. 2016. PMID: 26112391
-
Acute encephalitis syndrome surveillance, Kushinagar district, Uttar Pradesh, India, 2011-2012.Emerg Infect Dis. 2013;19(9):1361-7. doi: 10.3201/eid1909.121855. Emerg Infect Dis. 2013. PMID: 23965505 Free PMC article.
-
The Outbreaks of Acute Encephalitis Syndrome in Uttar Pradesh, India (1978-2020) and Its Effective Management: A Remarkable Public Health Success Story.Front Public Health. 2022 Feb 9;9:793268. doi: 10.3389/fpubh.2021.793268. eCollection 2021. Front Public Health. 2022. PMID: 35223759 Free PMC article. Review.
-
Systematic reviews of the effectiveness of day care for people with severe mental disorders: (1) acute day hospital versus admission; (2) vocational rehabilitation; (3) day hospital versus outpatient care.Health Technol Assess. 2001;5(21):1-75. doi: 10.3310/hta5210. Health Technol Assess. 2001. PMID: 11532238 Review.
Cited by
-
Oral disease-modifying antirheumatic drugs and immunosuppressants with antiviral potential, including SARS-CoV-2 infection: a review.Ther Adv Musculoskelet Dis. 2020 Sep 3;12:1759720X20947296. doi: 10.1177/1759720X20947296. eCollection 2020. Ther Adv Musculoskelet Dis. 2020. PMID: 32952617 Free PMC article.
-
Neuropsychiatric Symptoms and Tick-Borne Diseases.Curr Top Behav Neurosci. 2023;61:279-302. doi: 10.1007/7854_2022_406. Curr Top Behav Neurosci. 2023. PMID: 36512289
-
Drug Repurposing for Japanese Encephalitis Virus Infection by Systems Biology Methods.Molecules. 2018 Dec 18;23(12):3346. doi: 10.3390/molecules23123346. Molecules. 2018. PMID: 30567313 Free PMC article.
-
Recent advances in understanding Japanese encephalitis.F1000Res. 2019 Nov 13;8:F1000 Faculty Rev-1915. doi: 10.12688/f1000research.19693.1. eCollection 2019. F1000Res. 2019. PMID: 31781366 Free PMC article. Review.
-
Diagnosis and Management of Acute Encephalitis in Children.Indian J Pediatr. 2019 Jan;86(1):70-75. doi: 10.1007/s12098-018-2775-0. Epub 2018 Sep 19. Indian J Pediatr. 2019. PMID: 30232787 Review.
References
-
- Japanese encephalitis surveillance standards. Available from: http://www.path.org/files/WHO_surveillance_standards_JE.pdf. Accessed September 10, 2014.
-
- Economic Profile of Uttar Pradesh Available from: http://www.economywatch.com/stateprofiles/uttarpradesh/profile.htm. Accessed September 10, 2014.
-
- Roop Kumari, Pyare L Joshi A review of Japanese encephalitis in Uttar Pradesh, India. WHO South-East Asia Journal of Public Health 2012;1(4):374-395. Ministry of Health & Family Welfare, Government of India Operational Guidelines Japanese encephalitis vaccination in India September 2010. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical