Validation-verification of a highly effective, practical human testicular tissue in vitro culture-cryopreservation procedure aimed to optimize pre-freeze and post-thaw motility
- PMID: 26847133
- PMCID: PMC4818637
- DOI: 10.1007/s10815-016-0659-7
Validation-verification of a highly effective, practical human testicular tissue in vitro culture-cryopreservation procedure aimed to optimize pre-freeze and post-thaw motility
Abstract
Purpose: The aim of our paper was to validate a testicular biopsy procedure that simplifies handling, processing, and cryopreservation, while at the same time optimizes sperm motility before freezing and after thawing.
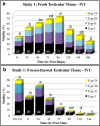
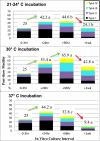
Methods: Two prospective studies were conducted to verify, optimize, and understand the virtues of pre-freeze testicular tissue IVC at different temperatures (21, 30, or 37 °C). Testicular tissue was obtained from clinical specimens designated for whole tissue cryopreservation (i.e., intact mass of tubules) and/or for fresh use in IVF-ICSI cycles. Whole testicular biopsy pieces (1-3 mm(3)) were diluted in glycerol containing freeze solutions, slow cooled to 4 °C and then rapidly frozen in LN2 vapor. Fresh and post-thaw testicular biopsy tissue were evaluated for changes in the quantity (%) and pattern of motility (I-IV: twitching to rapid progression, respectively) over a 1 week duration. The clinical effectiveness of IVC-cryopreserved whole testicular biopsy tissue was also validated analyzing fresh embryo transfers.
Results: More reliable recovery of motile testicular sperm was achieved using whole tissue freeze preservation combined with IVC (24-96 h) post-acquisition at an incubation temperature of 30 °C compared to ambient temperature (21 °C) or 37 °C. Up to 85 % of the pre-freeze motility was conserved post-thaw (+3 h) for easy ICSI selection. Sperm longevity was optimized to fresh tissue levels by implementing testicular biopsy sucrose dilution post-thaw. Favorable clinical outcomes were proven using frozen-thawed testicular biopsy sperm for ICSI.
Conclusions: By employing minimal tissue manipulation, integrating pre-freeze IVC processing at 30 °C and the freezing of whole testicular biopsy tissue, we have reduced the labor and improved the efficacy of processing testicular tissue for freeze-preservation and subsequent ICSI use.
Keywords: In vitro culture (IVC); Motility; Sperm cryopreservation; TESE; Testicular biopsy.
Figures


Similar articles
-
Optimal use of fresh and frozen-thawed testicular sperm for intracytoplasmic sperm injection in azoospermic patients.J Assist Reprod Genet. 2005 Dec;22(11-12):389-94. doi: 10.1007/s10815-005-7481-y. J Assist Reprod Genet. 2005. PMID: 16331535 Free PMC article.
-
Quality of cryopreserved testicular sperm in patients with obstructive and nonobstructive azoospermia.J Urol. 1999 May;161(5):1504-8. J Urol. 1999. PMID: 10210383 Clinical Trial.
-
Effects of cryopreservation on testicular sperm nuclear DNA fragmentation and its relationship with assisted conception outcome following ICSI with testicular spermatozoa.Reprod Biomed Online. 2003 Oct-Nov;7(4):449-55. doi: 10.1016/s1472-6483(10)61889-5. Reprod Biomed Online. 2003. PMID: 14656407
-
Cryopreservation of testicular tissue.Mol Cell Endocrinol. 2000 Nov 27;169(1-2):113-5. doi: 10.1016/s0303-7207(00)00363-4. Mol Cell Endocrinol. 2000. PMID: 11155942 Review.
-
[Advances in researches on cryopreservation of testicular spermatozoa].Zhonghua Nan Ke Xue. 2021 Jul;27(7):649-653. Zhonghua Nan Ke Xue. 2021. PMID: 34914235 Review. Chinese.
Cited by
-
Letter to Editor: We Need Standardized Guidelines for Laboratory Tissue Processing After Microsurgical Testicular Sperm Extraction.Reprod Sci. 2021 Aug;28(8):2071-2075. doi: 10.1007/s43032-021-00585-4. Epub 2021 Apr 19. Reprod Sci. 2021. PMID: 33871827 No abstract available.
-
Improvement of motility after culture of testicular spermatozoa: the effects of incubation timing and temperature.Transl Androl Urol. 2017 Apr;6(2):271-276. doi: 10.21037/tau.2017.03.43. Transl Androl Urol. 2017. PMID: 28540235 Free PMC article.
-
Long-term Preservation of Testicular Tissue Integrity and Viability Using Vitrification in the Endangered Black-Footed Ferret (Mustela Nigripes).Animals (Basel). 2020 Oct 13;10(10):1865. doi: 10.3390/ani10101865. Animals (Basel). 2020. PMID: 33066219 Free PMC article.
-
Transcriptomic Differences by RNA Sequencing for Evaluation of New Method for Long-Time In Vitro Culture of Cryopreserved Testicular Tissue for Oncologic Patients.Cells. 2024 Sep 13;13(18):1539. doi: 10.3390/cells13181539. Cells. 2024. PMID: 39329723 Free PMC article.
-
Comparative Transcriptomic Analyses for the Optimization of Thawing Regimes during Conventional Cryopreservation of Mature and Immature Human Testicular Tissue.Int J Mol Sci. 2023 Dec 22;25(1):214. doi: 10.3390/ijms25010214. Int J Mol Sci. 2023. PMID: 38203385 Free PMC article.
References
-
- Devroey P, Liu J, Nagy Z, et al. Normal fertilization of human oocytes after testicular sperm extraction and intracytoplasmic sperm injection. Fertil Steril. 1994;62:639–641. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

