The fully synthetic MAG-Tn3 therapeutic vaccine containing the tetanus toxoid-derived TT830-844 universal epitope provides anti-tumor immunity
- PMID: 26847142
- PMCID: PMC4779142
- DOI: 10.1007/s00262-016-1802-0
The fully synthetic MAG-Tn3 therapeutic vaccine containing the tetanus toxoid-derived TT830-844 universal epitope provides anti-tumor immunity
Abstract
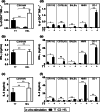
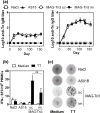
Malignant transformations are often associated with aberrant glycosylation processes that lead to the expression of new carbohydrate antigens at the surface of tumor cells. Of these carbohydrate antigens, the Tn antigen is particularly highly expressed in many carcinomas, especially in breast carcinoma. We designed MAG-Tn3, a fully synthetic vaccine based on three consecutive Tn moieties that are O-linked to a CD4+ T cell epitope, to induce anti-Tn antibody responses that could be helpful for therapeutic vaccination against cancer. To ensure broad coverage within the human population, the tetanus toxoid-derived peptide TT830-844 was selected as a T-helper epitope because it can bind to various HLA-DRB molecules. We showed that the MAG-Tn3 vaccine, which was formulated with the GSK proprietary immunostimulant AS15 and designed for human cancer therapy, is able to induce an anti-Tn antibody response in mice of various H-2 haplotypes, and this response correlates with the ability to induce a specific T cell response against the TT830-844 peptide. The universality of the TT830-844 peptide was extended to new H-2 and HLA-DRB molecules that were capable of binding this T cell epitope. Finally, the MAG-Tn3 vaccine was able to induce anti-Tn antibody responses in cynomolgus monkeys, which targeted Tn-expressing tumor cells and mediated tumor cell death both in vitro and in vivo. Thus, MAG-Tn3 is a highly promising anticancer vaccine that is currently under evaluation in a phase I clinical trial.
Keywords: Antibody response; Anticancer vaccine; MAG-Tn3; TT830-844; Universal epitope.
Conflict of interest statement
Anne-Laure Puaux and Catherine Gérard are employed by the GSK group of companies. Catherine Gérard declares stock ownership in the GSK group of companies, and Anne-Laure Puaux declares annual stock options. All other authors declare that they have no conflicts of interest.
Figures




Similar articles
-
The fully synthetic glycopeptide MAG-Tn3 therapeutic vaccine induces tumor-specific cytotoxic antibodies in breast cancer patients.Cancer Immunol Immunother. 2020 May;69(5):703-716. doi: 10.1007/s00262-020-02503-0. Epub 2020 Feb 7. Cancer Immunol Immunother. 2020. PMID: 32034426 Free PMC article. Clinical Trial.
-
Anti-tumor immunity provided by a synthetic multiple antigenic glycopeptide displaying a tri-Tn glycotope.J Immunol. 2001 Feb 15;166(4):2849-54. doi: 10.4049/jimmunol.166.4.2849. J Immunol. 2001. PMID: 11160353
-
A fully synthetic therapeutic vaccine candidate targeting carcinoma-associated Tn carbohydrate antigen induces tumor-specific antibodies in nonhuman primates.Cancer Res. 2004 Jul 15;64(14):4987-94. doi: 10.1158/0008-5472.CAN-04-0252. Cancer Res. 2004. PMID: 15256473
-
Approaches to Improve Chemically Defined Synthetic Peptide Vaccines.Front Immunol. 2018 Apr 26;9:884. doi: 10.3389/fimmu.2018.00884. eCollection 2018. Front Immunol. 2018. PMID: 29755468 Free PMC article. Review.
-
Recent development in carbohydrate-based cancer vaccines.Curr Opin Chem Biol. 2009 Dec;13(5-6):608-17. doi: 10.1016/j.cbpa.2009.08.010. Epub 2009 Sep 18. Curr Opin Chem Biol. 2009. PMID: 19766052 Free PMC article. Review.
Cited by
-
The fully synthetic glycopeptide MAG-Tn3 therapeutic vaccine induces tumor-specific cytotoxic antibodies in breast cancer patients.Cancer Immunol Immunother. 2020 May;69(5):703-716. doi: 10.1007/s00262-020-02503-0. Epub 2020 Feb 7. Cancer Immunol Immunother. 2020. PMID: 32034426 Free PMC article. Clinical Trial.
-
Bioinformatic, Biochemical, and Immunological Mining of MHC Class I Restricted T Cell Epitopes for a Marburg Nucleoprotein Microparticle Vaccine.Vaccines (Basel). 2024 Mar 18;12(3):322. doi: 10.3390/vaccines12030322. Vaccines (Basel). 2024. PMID: 38543955 Free PMC article.
-
Mining the Immunopeptidome for Antigenic Peptides in Cancer.Cancers (Basel). 2022 Oct 11;14(20):4968. doi: 10.3390/cancers14204968. Cancers (Basel). 2022. PMID: 36291752 Free PMC article. Review.
-
Targeting Tumor Glycans for Cancer Therapy: Successes, Limitations, and Perspectives.Cancers (Basel). 2022 Jan 27;14(3):645. doi: 10.3390/cancers14030645. Cancers (Basel). 2022. PMID: 35158915 Free PMC article. Review.
-
A T cell-targeted multi-antigen vaccine generates robust cellular and humoral immunity against SARS-CoV-2 infection.Mol Ther Methods Clin Dev. 2023 Sep 16;31:101110. doi: 10.1016/j.omtm.2023.101110. eCollection 2023 Dec 14. Mol Ther Methods Clin Dev. 2023. PMID: 37822719 Free PMC article.
References
-
- Mazal D, Lo-Man R, Bay S, Pritsch O, Deriaud E, Ganneau C, Medeiros A, Ubillos L, Obal G, Berois N, Bollati-Fogolin M, Leclerc C, Osinaga E. Monoclonal antibodies toward different Tn-amino acid backbones display distinct recognition patterns on human cancer cells. Implications for effective immuno-targeting of cancer. Cancer Immunol Immunother. 2013;62(6):1107–1122. doi: 10.1007/s00262-013-1425-7. - DOI - PMC - PubMed
-
- Lo-Man R, Bay S, Vichier-Guerre S, Deriaud E, Cantacuzene D, Leclerc C. A fully synthetic immunogen carrying a carcinoma-associated carbohydrate for active specific immunotherapy. Cancer Res. 1999;59(7):1520–1524. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

