Standardization of Weed Pollen Extracts, Japanese Hop and Mugwort, in Korea
- PMID: 26847293
- PMCID: PMC4740533
- DOI: 10.3349/ymj.2016.57.2.399
Standardization of Weed Pollen Extracts, Japanese Hop and Mugwort, in Korea
Abstract
Purpose: Japanese hop (Humulus spp.) and mugwort (Artemisia spp.) are notable causes of autumn pollinosis in East Asia. However, Japanese hop and mugwort pollen extracts, which are widely used for the diagnosis, have not been standardized. This study was performed to standardize Japanese hop and mugwort pollen extracts.
Materials and methods: Allergen extracts were prepared in a standardized way using locally collected Humulus japonicus and purchased Artemisia vulgaris pollens. The immunoglobulin E (IgE) reactivities of prepared extracts were compared with commercial extracts via IgE immunoblotting and inhibition analyses. Intradermal skin tests were performed to determine the bioequivalent allergy unit (BAU).
Results: The IgE reactive components of the extracts via IgE immunoblotting were similar to those of commercial extracts. A 11-kDa allergen showed the strongest IgE reactivity in Japanese hop, as did a 28-kDa allergen in mugwort pollen extracts. Allergenic potencies of the investigatory Japanese hop and mugwort extracts were essentially indistinguishable from the commercial ones. Sums of erythema of 50 mm by the intradermal skin test (ΣED50) were calculated to be 14.4th and 13.6th three-fold dilutions for Japanese hop and mugwort extracts, respectively. Therefore, the allergenic activity of the prepared extracts was 90827.4 BAU/mg for Japanese hop and 34412 BAU/mg for mugwort.
Conclusion: We produced Japanese hop and mugwort pollen extracts using a standardized method. Standardized Japanese hop and mugwort pollen extracts will facilitate the production of improved diagnostic and immunotherapeutic reagents.
Keywords: Allergen standardization; Japanese hop; mugwort.
Conflict of interest statement
The authors have no financial conflicts of interest.
Figures




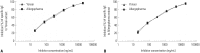

Similar articles
-
IgE Cross-Reactivity between Humulus japonicus and Humulus lupulus.Yonsei Med J. 2018 Sep;59(7):852-856. doi: 10.3349/ymj.2018.59.7.852. Yonsei Med J. 2018. PMID: 30091318 Free PMC article.
-
Evidence of Hop Japanese pollinosis in Korea: IgE sensitization and identification of allergenic components.J Allergy Clin Immunol. 1997 Oct;100(4):475-9. doi: 10.1016/s0091-6749(97)70138-6. J Allergy Clin Immunol. 1997. PMID: 9338540
-
IgE crossreactivity between mugwort pollen (Artemisia vulgaris) and hazelnut (Abellana nux) in sera from patients with sensitivity to both extracts.Clin Exp Allergy. 1997 Oct;27(10):1203-11. Clin Exp Allergy. 1997. PMID: 9383261
-
Emerging Hop Japanese Pollinosis in Asia.Curr Protein Pept Sci. 2022;23(11):714-720. doi: 10.2174/1389203723666220603155320. Curr Protein Pept Sci. 2022. PMID: 35658882 Review.
-
Pollen-food syndromes associated with weed pollinosis: an update from the molecular point of view.Allergy. 2006 Apr;61(4):461-76. doi: 10.1111/j.1398-9995.2006.00994.x. Allergy. 2006. PMID: 16512809 Review.
Cited by
-
Allergens of Regional Importance in Korea.Front Allergy. 2021 Mar 5;2:652275. doi: 10.3389/falgy.2021.652275. eCollection 2021. Front Allergy. 2021. PMID: 35386990 Free PMC article. Review.
-
Validation of PROTIA™ Allergy-Q 64 Atopy® as a Specific IgE Measurement Assay for 10 Major Allergen Components.Allergy Asthma Immunol Res. 2019 May;11(3):422-432. doi: 10.4168/aair.2019.11.3.422. Allergy Asthma Immunol Res. 2019. PMID: 30912330 Free PMC article.
-
IgE Cross-Reactivity between Humulus japonicus and Humulus lupulus.Yonsei Med J. 2018 Sep;59(7):852-856. doi: 10.3349/ymj.2018.59.7.852. Yonsei Med J. 2018. PMID: 30091318 Free PMC article.
-
Stability of extracts from pollens of allergenic importance in Korea.Korean J Intern Med. 2020 Jan;35(1):222-230. doi: 10.3904/kjim.2018.211. Epub 2019 Sep 20. Korean J Intern Med. 2020. PMID: 31534112 Free PMC article.
-
Application of Pauson-Khand reaction in the total synthesis of terpenes.RSC Adv. 2021 Nov 29;11(61):38325-38373. doi: 10.1039/d1ra05673e. eCollection 2021 Nov 29. RSC Adv. 2021. PMID: 35493249 Free PMC article. Review.
References
-
- Park HS, Coi SY, Nahm DH, Kim HY. Revival of Hop Japanese pollinosis in asthmatic subjects in Kyungki area. J Asthma Allergy Clin Immunol. 1998;18:52–60.
-
- Weber RW. On the cover. Hop. Ann Allergy Asthma Immunol. 2008;100:A4. - PubMed
-
- Hong CS, Hwang Y, Oh SH, Kim HJ, Huh KB, Lee SY. Survey of the airborne pollens in Seoul, Korea. Yonsei Med J. 1986;27:114–120. - PubMed
-
- Park HS, Nahm DH, Suh CH, Lee SM, Choi SY, Jung KS, et al. Evidence of Hop Japanese pollinosis in Korea: IgE sensitization and identification of allergenic components. J Allergy Clin Immunol. 1997;100:475–479. - PubMed
-
- Yoon YW, Lee MK, Park HS, Park SS, Hong CS. The skin test reactivity and the level of the total IgE in the allergic patients. Allergy. 1989;9:385–398.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

