Is Tamsulosin 0.2 mg Effective and Safe as a First-Line Treatment Compared with Other Alpha Blockers?: A Meta-Analysis and a Moderator Focused Study
- PMID: 26847294
- PMCID: PMC4740534
- DOI: 10.3349/ymj.2016.57.2.407
Is Tamsulosin 0.2 mg Effective and Safe as a First-Line Treatment Compared with Other Alpha Blockers?: A Meta-Analysis and a Moderator Focused Study
Abstract
Purpose: Tamsulosin 0.2 mg is used widely in Asian people, but the low dose has been studied less than tamsulosin 0.4 mg or other alpha blockers of standard dose. This study investigated the efficacy and safety of tamsulosin 0.2 mg by a meta-analysis and meta-regression.
Materials and methods: We conducted a meta-analysis of efficacy of tamsulosin 0.2 mg using International Prostate Symptom Score (IPSS), maximal urinary flow rate (Qmax), post-voided residual volume (PVR), and quality of life (QoL). Safety was analyzed using adverse events. Relevant studies were searched using MEDLINE, EMBASE, and Cochrane library from January 1980 to June 2013.
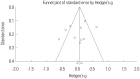
Results: Ten studies were included with a total sample size of 1418 subjects [722 tamsulosin 0.2 mg group and 696 other alpha-blockers (terazosin, doxazosin, naftopidil, silodosin) group]. Study duration ranged from 4 to 24 weeks. The pooled overall standardized mean differences (SMD) in the mean change of IPSS from baseline for the tamsulosin group versus the control group was 0.02 [95% confidence interval (CI); -0.20, 0.25]. The pooled overall SMD in the mean change of QoL from baseline for the tamsulosin group versus the control group was 0.16 (95% CI; -0.16, 0.48). The regression analysis with the continuous variables (number of patients, study duration) revealed no significance in all outcomes as IPSS, QoL, and Qmax.
Conclusion: This study clarifies that tamsulosin 0.2 mg has similar efficacy and fewer adverse events compared with other alpha-blockers as an initial treatment strategy for men with lower urinary tract symptoms.
Keywords: Prostatic hyperplasia; alpha blockers; tamsulosin.
Conflict of interest statement
The authors have no financial conflicts of interest.
Figures






References
-
- Chapple CR. BHP Disease Management. Introduction and concluding remarks. Eur Urol. 1999;36(Suppl 3):1–6. - PubMed
-
- Djavan B, Chapple C, Milani S, Marberger M. State of the art on the efficacy and tolerability of alpha1-adrenoceptor antagonists in patients with lower urinary tract symptoms suggestive of benign prostatic hyperplasia. Urology. 2004;64:1081–1088. - PubMed
-
- Lee E. Comparison of tamsulosin and finasteride for lower urinary tract symptoms associated with benign prostatic hyperplasia in Korean patients. J Int Med Res. 2002;30:584–590. - PubMed
-
- Lee E, Lee C. Clinical comparison of selective and non-selective alpha 1A-adrenoreceptor antagonists in benign prostatic hyperplasia: studies on tamsulosin in a fixed dose and terazosin in increasing doses. Br J Urol. 1997;80:606–611. - PubMed
-
- Li NC, Chen S, Yang XH, Du LD, Wang JY, Na YQ Beijing Tamsulosin Study Group. Efficacy of low-dose tamsulosin in chinese patients with symptomatic benign prostatic hyperplasia. Clin Drug Investig. 2003;23:781–787. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

