Limited role of culture conversion for decision-making in individual patient care and for advancing novel regimens to confirmatory clinical trials
- PMID: 26847437
- PMCID: PMC4743210
- DOI: 10.1186/s12916-016-0565-y
Limited role of culture conversion for decision-making in individual patient care and for advancing novel regimens to confirmatory clinical trials
Erratum in
-
Erratum to: Limited role of culture conversion for decision-making in individual patient care and for advancing novel regimens to confirmatory clinical trials.BMC Med. 2016 Feb 23;14:36. doi: 10.1186/s12916-016-0585-7. BMC Med. 2016. PMID: 26905218 Free PMC article. No abstract available.
Abstract
Background: Despite recent increased clinical trials activity, no regimen has proved able to replace the standard 6-month regimen for drug-sensitive tuberculosis. Understanding the relationship between microbiological markers measured during treatment and long-term clinical outcomes is critical to evaluate their usefulness for decision-making for both individual patient care and for advancing novel regimens into time-consuming and expensive pivotal phase III trials.
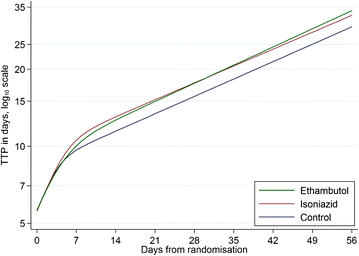
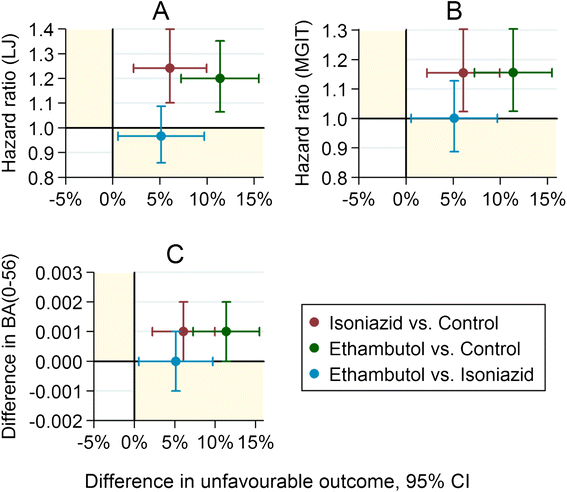
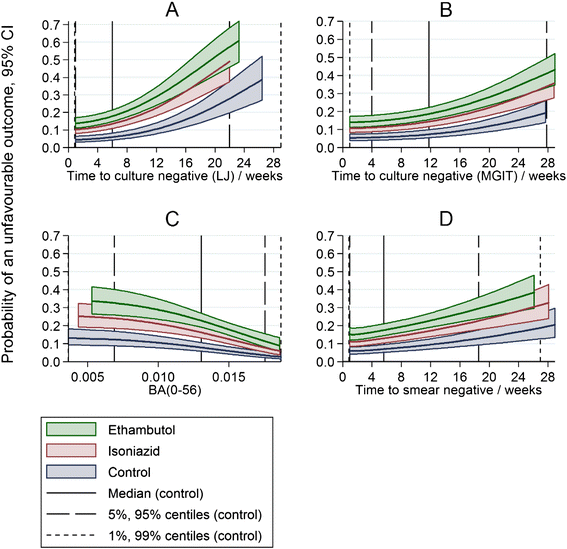
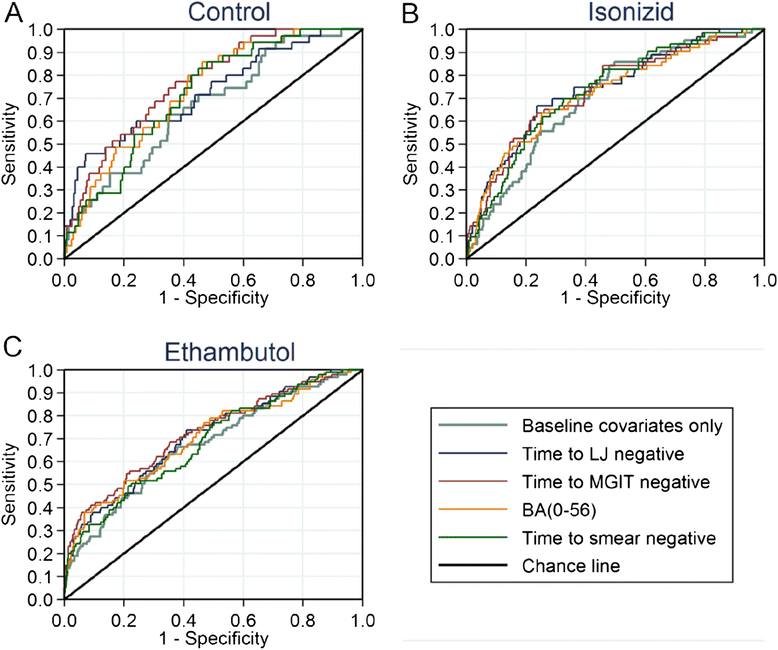
Methods: Using data from the randomized controlled phase III trial REMoxTB, we evaluated sputum-based markers of speed of clearance of bacilli: time to smear negative status; time to culture negative status on LJ or in MGIT; daily rate of change of log10(TTP) to day 56; and smear or culture results at weeks 6, 8 or 12; as individual- and trial-level surrogate endpoints for long-term clinical outcome.
Results: Time to culture negative status on LJ or in MGIT, time to smear negative status and daily rate of change in log10(TTP) were each independent predictors of clinical outcome, adjusted for treatment (p <0.001). However, discrimination between low and high risk patients, as measured by the c-statistic, was modest and not much higher than the reference model adjusted for BMI, history of smoking, HIV status, cavitation, gender and MGIT TTP.
Conclusions: Culture conversion during treatment for tuberculosis, however measured, has only a limited role in decision-making for advancing regimens into phase III trials or in predicting the outcome of treatment for individual patients. REMoxTB ClinicalTrials.gov number: NCT00864383.
Figures
Similar articles
-
Shortened treatment regimens versus the standard regimen for drug-sensitive pulmonary tuberculosis.Cochrane Database Syst Rev. 2019 Dec 12;12(12):CD012918. doi: 10.1002/14651858.CD012918.pub2. Cochrane Database Syst Rev. 2019. PMID: 31828771 Free PMC article.
-
A comparison of liquid and solid culture for determining relapse and durable cure in phase III TB trials for new regimens.BMC Med. 2017 Nov 24;15(1):207. doi: 10.1186/s12916-017-0955-9. BMC Med. 2017. PMID: 29169355 Free PMC article.
-
Lipoarabinomannan in sputum to detect bacterial load and treatment response in patients with pulmonary tuberculosis: Analytic validation and evaluation in two cohorts.PLoS Med. 2019 Apr 12;16(4):e1002780. doi: 10.1371/journal.pmed.1002780. eCollection 2019 Apr. PLoS Med. 2019. PMID: 30978194 Free PMC article.
-
Predictors of delayed culture conversion among Ugandan patients.BMC Infect Dis. 2017 Apr 24;17(1):299. doi: 10.1186/s12879-017-2335-7. BMC Infect Dis. 2017. PMID: 28438118 Free PMC article. Clinical Trial.
-
Folic acid supplementation and malaria susceptibility and severity among people taking antifolate antimalarial drugs in endemic areas.Cochrane Database Syst Rev. 2022 Feb 1;2(2022):CD014217. doi: 10.1002/14651858.CD014217. Cochrane Database Syst Rev. 2022. PMID: 36321557 Free PMC article.
Cited by
-
Design principles to assemble drug combinations for effective tuberculosis therapy using interpretable pairwise drug response measurements.Cell Rep Med. 2022 Sep 20;3(9):100737. doi: 10.1016/j.xcrm.2022.100737. Epub 2022 Sep 8. Cell Rep Med. 2022. PMID: 36084643 Free PMC article.
-
Resolution of tuberculosis blood RNA signatures fails to discriminate persistent sputum culture positivity after 8 weeks of anti-tuberculous treatment.Eur Respir J. 2024 Nov 21;64(5):2400457. doi: 10.1183/13993003.00457-2024. Print 2024 Nov. Eur Respir J. 2024. PMID: 39190790 Free PMC article.
-
A new trial design to accelerate tuberculosis drug development: the Phase IIC Selection Trial with Extended Post-treatment follow-up (STEP).BMC Med. 2016 Mar 23;14:51. doi: 10.1186/s12916-016-0597-3. BMC Med. 2016. PMID: 27004726 Free PMC article. Clinical Trial.
-
Systematic assessment of clinical and bacteriological markers for tuberculosis reveals discordance and inaccuracy of symptom-based diagnosis for treatment response monitoring.Front Med (Lausanne). 2022 Oct 28;9:992451. doi: 10.3389/fmed.2022.992451. eCollection 2022. Front Med (Lausanne). 2022. PMID: 36419786 Free PMC article.
-
Shortened treatment regimens versus the standard regimen for drug-sensitive pulmonary tuberculosis.Cochrane Database Syst Rev. 2019 Dec 12;12(12):CD012918. doi: 10.1002/14651858.CD012918.pub2. Cochrane Database Syst Rev. 2019. PMID: 31828771 Free PMC article.
References
-
- World Health Organization (WHO) Global tuberculosis report 2014. Geneva: WHO; 2014.
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

