Emerging evidence for beneficial macrophage functions in atherosclerosis and obesity-induced insulin resistance
- PMID: 26847458
- PMCID: PMC4803808
- DOI: 10.1007/s00109-016-1385-4
Emerging evidence for beneficial macrophage functions in atherosclerosis and obesity-induced insulin resistance
Abstract
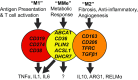
The discovery that obesity promotes macrophage accumulation in visceral fat led to the emergence of a new field of inquiry termed "immunometabolism". This broad field of study was founded on the premise that inflammation and the corresponding increase in macrophage number and activity was a pathologic feature of metabolic diseases. There is abundant data in both animal and human studies that supports this assertation. Established adverse effects of inflammation in visceral fat include decreased glucose and fatty acid uptake, inhibition of insulin signaling, and ectopic triglyceride accumulation. Likewise, in the atherosclerotic plaque, macrophage accumulation and activation results in plaque expansion and destabilization. Despite these facts, there is an accumulating body of evidence that macrophages also have beneficial functions in both atherosclerosis and visceral obesity. Potentially beneficial functions that are common to these different contexts include the regulation of efferocytosis, lipid buffering, and anti-inflammatory effects. Autophagy, the process by which cytoplasmic contents are delivered to the lysosome for degradation, is integral to many of these protective biologic functions. The macrophage utilizes autophagy as a molecular tool to maintain tissue integrity and homeostasis at baseline (e.g., bone growth) and in the face of ongoing metabolic insults (e.g., fasting, hypercholesterolemia, obesity). Herein, we highlight recent evidence demonstrating that abrogation of certain macrophage functions, in particular autophagy, exacerbates both atherosclerosis and obesity-induced insulin resistance. Insulin signaling through mammalian target of rapamycin (mTOR) is a crucial regulatory node that links nutrient availability to macrophage autophagic flux. A more precise understanding of the metabolic substrates and triggers for macrophage autophagy may allow therapeutic manipulation of this pathway. These observations underscore the complexity of the field "immunometabolism", validate its importance, and raise many fascinating and important questions for future study.
Keywords: Atherosclerosis; Autophagy; Cholesterol; Insulin resistance; Macrophage; Visceral adipose; mTOR.
Figures

Similar articles
-
Cellular and molecular players in adipose tissue inflammation in the development of obesity-induced insulin resistance.Biochim Biophys Acta. 2014 Mar;1842(3):446-62. doi: 10.1016/j.bbadis.2013.05.017. Epub 2013 May 22. Biochim Biophys Acta. 2014. PMID: 23707515 Free PMC article. Review.
-
MicroRNA-761 modulates foam cell formation and inflammation through autophagy in the progression of atherosclerosis.Mol Cell Biochem. 2020 Nov;474(1-2):135-146. doi: 10.1007/s11010-020-03839-y. Epub 2020 Aug 8. Mol Cell Biochem. 2020. PMID: 32772311
-
IgE Contributes to Atherosclerosis and Obesity by Affecting Macrophage Polarization, Macrophage Protein Network, and Foam Cell Formation.Arterioscler Thromb Vasc Biol. 2020 Mar;40(3):597-610. doi: 10.1161/ATVBAHA.119.313744. Epub 2020 Jan 30. Arterioscler Thromb Vasc Biol. 2020. PMID: 31996021 Free PMC article.
-
The immune-metabolic regulatory roles of epoxyeicosatrienoic acids on macrophages phenotypic plasticity in obesity-related insulin resistance.Prostaglandins Other Lipid Mediat. 2018 Nov;139:36-40. doi: 10.1016/j.prostaglandins.2018.10.003. Epub 2018 Oct 5. Prostaglandins Other Lipid Mediat. 2018. PMID: 30296488 Review.
-
Beneficial metabolic effects of rapamycin are associated with enhanced regulatory cells in diet-induced obese mice.PLoS One. 2014 Apr 7;9(4):e92684. doi: 10.1371/journal.pone.0092684. eCollection 2014. PLoS One. 2014. PMID: 24710396 Free PMC article.
Cited by
-
Triglyceride Glucose Index and Prognosis of Patients With Ischemic Stroke.Front Neurol. 2020 Jun 10;11:456. doi: 10.3389/fneur.2020.00456. eCollection 2020. Front Neurol. 2020. PMID: 32587566 Free PMC article.
-
A Lipophilic Fucoxanthin-Rich Phaeodactylum tricornutum Extract Ameliorates Effects of Diet-Induced Obesity in C57BL/6J Mice.Nutrients. 2019 Apr 6;11(4):796. doi: 10.3390/nu11040796. Nutrients. 2019. PMID: 30959933 Free PMC article.
-
Activated macrophages control human adipocyte mitochondrial bioenergetics via secreted factors.Mol Metab. 2017 Oct;6(10):1226-1239. doi: 10.1016/j.molmet.2017.07.008. Epub 2017 Jul 19. Mol Metab. 2017. PMID: 29031722 Free PMC article.
-
Body mass index, as a novel predictor of hepatocellular carcinoma patients treated with Anti-PD-1 immunotherapy.Front Med (Lausanne). 2022 Sep 20;9:981001. doi: 10.3389/fmed.2022.981001. eCollection 2022. Front Med (Lausanne). 2022. PMID: 36203764 Free PMC article.
-
Silencing MR-1 attenuates atherosclerosis in ApoE-/- mice induced by angiotensin II through FAK-Akt-mTOR-NF-kappaB signaling pathway.Korean J Physiol Pharmacol. 2018 Mar;22(2):127-134. doi: 10.4196/kjpp.2018.22.2.127. Epub 2018 Feb 23. Korean J Physiol Pharmacol. 2018. PMID: 29520165 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

