Chimpanzee Adenovirus Vaccine Provides Multispecies Protection against Rift Valley Fever
- PMID: 26847478
- PMCID: PMC4742904
- DOI: 10.1038/srep20617
Chimpanzee Adenovirus Vaccine Provides Multispecies Protection against Rift Valley Fever
Abstract
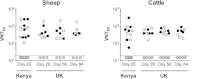
Rift Valley Fever virus (RVFV) causes recurrent outbreaks of acute life-threatening human and livestock illness in Africa and the Arabian Peninsula. No licensed vaccines are currently available for humans and those widely used in livestock have major safety concerns. A 'One Health' vaccine development approach, in which the same vaccine is co-developed for multiple susceptible species, is an attractive strategy for RVFV. Here, we utilized a replication-deficient chimpanzee adenovirus vaccine platform with an established human and livestock safety profile, ChAdOx1, to develop a vaccine for use against RVFV in both livestock and humans. We show that single-dose immunization with ChAdOx1-GnGc vaccine, encoding RVFV envelope glycoproteins, elicits high-titre RVFV-neutralizing antibody and provides solid protection against RVFV challenge in the most susceptible natural target species of the virus-sheep, goats and cattle. In addition we demonstrate induction of RVFV-neutralizing antibody by ChAdOx1-GnGc vaccination in dromedary camels, further illustrating the potency of replication-deficient chimpanzee adenovirus vaccine platforms. Thus, ChAdOx1-GnGc warrants evaluation in human clinical trials and could potentially address the unmet human and livestock vaccine needs.
Figures
Similar articles
-
Immunogenicity and efficacy of a chimpanzee adenovirus-vectored Rift Valley fever vaccine in mice.Virol J. 2013 Dec 5;10:349. doi: 10.1186/1743-422X-10-349. Virol J. 2013. PMID: 24304565 Free PMC article.
-
A single immunization with MVA expressing GnGc glycoproteins promotes epitope-specific CD8+-T cell activation and protects immune-competent mice against a lethal RVFV infection.PLoS Negl Trop Dis. 2013 Jul 11;7(7):e2309. doi: 10.1371/journal.pntd.0002309. Print 2013. PLoS Negl Trop Dis. 2013. PMID: 23875044 Free PMC article.
-
Single-dose of a replication-competent adenovirus-vectored vaccine provides sterilizing protection against Rift Valley fever virus challenge.Front Immunol. 2022 Nov 11;13:907675. doi: 10.3389/fimmu.2022.907675. eCollection 2022. Front Immunol. 2022. PMID: 36439179 Free PMC article.
-
Single-cycle replicable Rift Valley fever virus mutants as safe vaccine candidates.Virus Res. 2016 May 2;216:55-65. doi: 10.1016/j.virusres.2015.05.012. Epub 2015 May 27. Virus Res. 2016. PMID: 26022573 Free PMC article. Review.
-
Rift Valley fever vaccines: an overview of the safety and efficacy of the live-attenuated MP-12 vaccine candidate.Expert Rev Vaccines. 2017 Jun;16(6):601-611. doi: 10.1080/14760584.2017.1321482. Epub 2017 May 2. Expert Rev Vaccines. 2017. PMID: 28425834 Free PMC article. Review.
Cited by
-
Humoral immunity to phlebovirus infection.Ann N Y Acad Sci. 2023 Dec;1530(1):23-31. doi: 10.1111/nyas.15080. Epub 2023 Nov 7. Ann N Y Acad Sci. 2023. PMID: 37936483 Free PMC article. Review.
-
Identification and Immunogenicity of African Swine Fever Virus Antigens.Front Immunol. 2019 Jun 19;10:1318. doi: 10.3389/fimmu.2019.01318. eCollection 2019. Front Immunol. 2019. PMID: 31275307 Free PMC article.
-
Experimental Infection of Calves by Two Genetically-Distinct Strains of Rift Valley Fever Virus.Viruses. 2016 May 23;8(5):145. doi: 10.3390/v8050145. Viruses. 2016. PMID: 27223298 Free PMC article.
-
Vaccines: Underlying Principles of Design and Testing.Clin Pharmacol Ther. 2021 Apr;109(4):987-999. doi: 10.1002/cpt.2207. Epub 2021 Mar 11. Clin Pharmacol Ther. 2021. PMID: 33705574 Free PMC article.
-
Humoral Immunogenicity and Efficacy of a Single Dose of ChAdOx1 MERS Vaccine Candidate in Dromedary Camels.Sci Rep. 2019 Nov 8;9(1):16292. doi: 10.1038/s41598-019-52730-4. Sci Rep. 2019. PMID: 31705137 Free PMC article.
References
-
- Daubney R., Hudson J. R. & Granham P. C. Enzootic Hepatitis or Rift Valley fever: an undescribed virus disease of sheep, cattle and man from East Africa Journal of Pathology and Bacteriology 34, 545–579 (1931).
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

