Hypoxia: The Force that Drives Chronic Kidney Disease
- PMID: 26847481
- PMCID: PMC4851450
- DOI: 10.3121/cmr.2015.1282
Hypoxia: The Force that Drives Chronic Kidney Disease
Abstract
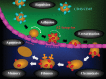
In the United States the prevalence of end-stage renal disease (ESRD) reached epidemic proportions in 2012 with over 600,000 patients being treated. The rates of ESRD among the elderly are disproportionally high. Consequently, as life expectancy increases and the baby-boom generation reaches retirement age, the already heavy burden imposed by ESRD on the US health care system is set to increase dramatically. ESRD represents the terminal stage of chronic kidney disease (CKD). A large body of evidence indicating that CKD is driven by renal tissue hypoxia has led to the development of therapeutic strategies that increase kidney oxygenation and the contention that chronic hypoxia is the final common pathway to end-stage renal failure. Numerous studies have demonstrated that one of the most potent means by which hypoxic conditions within the kidney produce CKD is by inducing a sustained inflammatory attack by infiltrating leukocytes. Indispensable to this attack is the acquisition by leukocytes of an adhesive phenotype. It was thought that this process resulted exclusively from leukocytes responding to cytokines released from ischemic renal endothelium. However, recently it has been demonstrated that leukocytes also become activated independent of the hypoxic response of endothelial cells. It was found that this endothelium-independent mechanism involves leukocytes directly sensing hypoxia and responding by transcriptional induction of the genes that encode the β2-integrin family of adhesion molecules. This induction likely maintains the long-term inflammation by which hypoxia drives the pathogenesis of CKD. Consequently, targeting these transcriptional mechanisms would appear to represent a promising new therapeutic strategy.
Keywords: CD43; CD45; Gene transcription; Hypoxia; Kidney disease; Leukocyte adhesion; β2-integrins.
© 2016 Marshfield Clinic.
Figures

References
-
- Fresenius Medical Care AG & Co. ESRD patients in 2012. A global perspective. Available at: http://www.vision-fmc.com/files/pdf_2/ESRD_Patients_2012.pdf Accessed February 26, 2015.
-
- Moeller S, Gioberge S, Brown G. ESRD patients in 2001: global overview of patients, treatment modalities and development trends. Nephrol Dial Transplant 2002;17:2071–2076. - PubMed
-
- Alebiosu CO, Ayodele OE. The global burden of chronic kidney disease and the way forward. Ethn Dis 2005;15:418–423. - PubMed
-
- National Institutes of Health, National Institute of Diabetes & Digestive & Kidney Diseases, Division of Kidny, Urologic, & Hematologic Diseases. 2013 USRDS Annual Data Report. Volume 2. Atlas of end-stage renal disease in the United States. Available at: http://www.usrds.org/2013/pdf/v2_00_intro_13.pdf Accessed February 26 2015
-
- Coresh J, Astor BC, Greene T, Eknoyan G, Levey AS. Prevalence of chronic kidney disease and decreased kidney function in the adult US population: Third National Health and Nutrition Examination Survey. Am J Kidney Dis 2003;41:1–12. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous
