KCTD12 Regulates Colorectal Cancer Cell Stemness through the ERK Pathway
- PMID: 26847701
- PMCID: PMC4742820
- DOI: 10.1038/srep20460
KCTD12 Regulates Colorectal Cancer Cell Stemness through the ERK Pathway
Abstract
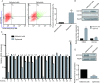
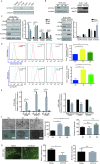
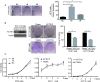
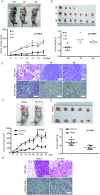
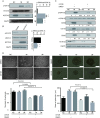
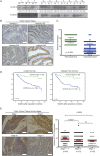
Targeting cancer stem cells (CSCs) in colorectal cancer (CRC) remains a difficult problem, as the regulation of CSCs in CRC is poorly understood. Here we demonstrated that KCTD12, potassium channel tetramerization domain containing 12, is down-regulated in the CSC-like cells of CRC. The silencing of endogenous KCTD12 and the overexpression of ectopic KCTD12 dramatically enhances and represses CRC cell stemness, respectively, as assessed in vitro and in vivo using a colony formation assay, a spheroid formation assay and a xenograft tumor model. Mechanistically, KCTD12 suppresses CRC cell stemness markers, such as CD44, CD133 and CD29, by inhibiting the ERK pathway, as the ERK1/2 inhibitor U0126 abolishes the increase in expression of CRC cell stemness markers induced by the down-regulation of KCTD12. Indeed, a decreased level of KCTD12 is detected in CRC tissues compared with their adjacent normal tissues and is an independent prognostic factor for poor overall and disease free survival in patients with CRC (p = 0.007). Taken together, this report reveals that KCTD12 is a novel regulator of CRC cell stemness and may serve as a novel prognostic marker and therapeutic target for patients with CRC.
Figures

Similar articles
-
Prostaglandin E2 Promotes Colorectal Cancer Stem Cell Expansion and Metastasis in Mice.Gastroenterology. 2015 Dec;149(7):1884-1895.e4. doi: 10.1053/j.gastro.2015.07.064. Epub 2015 Aug 7. Gastroenterology. 2015. PMID: 26261008 Free PMC article.
-
Maintenance of cancer stemness by miR-196b-5p contributes to chemoresistance of colorectal cancer cells via activating STAT3 signaling pathway.Oncotarget. 2017 Jul 25;8(30):49807-49823. doi: 10.18632/oncotarget.17971. Oncotarget. 2017. PMID: 28591704 Free PMC article.
-
Lgr5 promotes cancer stemness and confers chemoresistance through ABCB1 in colorectal cancer.Biomed Pharmacother. 2013 Oct;67(8):791-9. doi: 10.1016/j.biopha.2013.08.001. Epub 2013 Aug 23. Biomed Pharmacother. 2013. PMID: 24138824
-
Cancer stem cells in colorectal cancer: a review.J Clin Pathol. 2018 Feb;71(2):110-116. doi: 10.1136/jclinpath-2017-204739. Epub 2017 Sep 23. J Clin Pathol. 2018. PMID: 28942428 Review.
-
Colorectal Cancer Stem Cell Biomarkers: Biological Traits and Prognostic Insights.Curr Pharm Des. 2024;30(18):1386-1397. doi: 10.2174/0113816128291321240329050945. Curr Pharm Des. 2024. PMID: 38623972 Review.
Cited by
-
Prospective evaluation of the lymph node proteome in dogs with multicentric lymphoma supplemented with sulforaphane.J Vet Intern Med. 2020 Sep;34(5):2036-2047. doi: 10.1111/jvim.15898. Epub 2020 Sep 14. J Vet Intern Med. 2020. PMID: 32926463 Free PMC article.
-
Diacerein treatment prevents colitis-associated cancer in mice.World J Clin Oncol. 2020 Sep 24;11(9):732-746. doi: 10.5306/wjco.v11.i9.732. World J Clin Oncol. 2020. PMID: 33033695 Free PMC article.
-
DRUM: Inference of Disease-Associated m6A RNA Methylation Sites From a Multi-Layer Heterogeneous Network.Front Genet. 2019 Apr 3;10:266. doi: 10.3389/fgene.2019.00266. eCollection 2019. Front Genet. 2019. PMID: 31001320 Free PMC article.
-
Cancer stem cells in colorectal cancer and the association with chemotherapy resistance.Med Oncol. 2021 Mar 18;38(4):43. doi: 10.1007/s12032-021-01488-9. Med Oncol. 2021. PMID: 33738588 Review.
-
Proteomic Signatures of Diffuse and Intestinal Subtypes of Gastric Cancer.Cancers (Basel). 2021 Nov 25;13(23):5930. doi: 10.3390/cancers13235930. Cancers (Basel). 2021. PMID: 34885041 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

