Long-Term Morphological and Microarchitectural Stability of Tissue-Engineered, Patient-Specific Auricles In Vivo
- PMID: 26847742
- PMCID: PMC4800266
- DOI: 10.1089/ten.TEA.2015.0323
Long-Term Morphological and Microarchitectural Stability of Tissue-Engineered, Patient-Specific Auricles In Vivo
Abstract
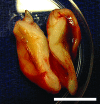
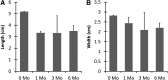
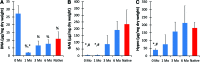
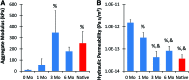
Current techniques for autologous auricular reconstruction produce substandard ear morphologies with high levels of donor-site morbidity, whereas alloplastic implants demonstrate poor biocompatibility. Tissue engineering, in combination with noninvasive digital photogrammetry and computer-assisted design/computer-aided manufacturing technology, offers an alternative method of auricular reconstruction. Using this method, patient-specific ears composed of collagen scaffolds and auricular chondrocytes have generated auricular cartilage with great fidelity following 3 months of subcutaneous implantation, however, this short time frame may not portend long-term tissue stability. We hypothesized that constructs developed using this technique would undergo continued auricular cartilage maturation without degradation during long-term (6 month) implantation. Full-sized, juvenile human ear constructs were injection molded from high-density collagen hydrogels encapsulating juvenile bovine auricular chondrocytes and implanted subcutaneously on the backs of nude rats for 6 months. Upon explantation, constructs retained overall patient morphology and displayed no evidence of tissue necrosis. Limited contraction occurred in vivo, however, no significant change in size was observed beyond 1 month. Constructs at 6 months showed distinct auricular cartilage microstructure, featuring a self-assembled perichondrial layer, a proteoglycan-rich bulk, and rounded cellular lacunae. Verhoeff's staining also revealed a developing elastin network comparable to native tissue. Biochemical measurements for DNA, glycosaminoglycan, and hydroxyproline content and mechanical properties of aggregate modulus and hydraulic permeability showed engineered tissue to be similar to native cartilage at 6 months. Patient-specific auricular constructs demonstrated long-term stability and increased cartilage tissue development during extended implantation, and offer a potential tissue-engineered solution for the future of auricular reconstructions.
Figures


Similar articles
-
High-fidelity tissue engineering of patient-specific auricles for reconstruction of pediatric microtia and other auricular deformities.PLoS One. 2013;8(2):e56506. doi: 10.1371/journal.pone.0056506. Epub 2013 Feb 20. PLoS One. 2013. PMID: 23437148 Free PMC article.
-
Optimizing cell sourcing for clinical translation of tissue engineered ears.Biofabrication. 2016 Dec 5;9(1):015004. doi: 10.1088/1758-5090/9/1/015004. Biofabrication. 2016. PMID: 27917821
-
Successful creation of tissue-engineered autologous auricular cartilage in an immunocompetent large animal model.Tissue Eng Part A. 2014 Jan;20(1-2):303-12. doi: 10.1089/ten.TEA.2013.0150. Epub 2013 Oct 4. Tissue Eng Part A. 2014. PMID: 23980800
-
[Regenerative medicine of tissue engineering: auricular cartilage regeneration and functional reconstruction].Lin Chuang Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2019 Jun;33(6):567-571. doi: 10.13201/j.issn.1001-1781.2019.06.024. Lin Chuang Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2019. PMID: 31163539 Review. Chinese.
-
Quantitative evaluation of mechanical properties in tissue-engineered auricular cartilage.Tissue Eng Part B Rev. 2014 Feb;20(1):17-27. doi: 10.1089/ten.TEB.2013.0117. Epub 2013 Jul 3. Tissue Eng Part B Rev. 2014. PMID: 23678981 Review.
Cited by
-
Three-Dimensional-Printed External Scaffolds Mitigate Loss of Volume and Topography in Engineered Elastic Cartilage Constructs.Cartilage. 2021 Dec;13(2_suppl):1780S-1789S. doi: 10.1177/19476035211049556. Epub 2021 Oct 12. Cartilage. 2021. PMID: 34636646 Free PMC article.
-
Biochemical and biomechanical characterization of the cervical, thoracic, and lumbar facet joint cartilage in the Yucatan minipig.J Biomech. 2022 Sep;142:111238. doi: 10.1016/j.jbiomech.2022.111238. Epub 2022 Jul 30. J Biomech. 2022. PMID: 35933954 Free PMC article.
-
The application and progress of stem cells in auricular cartilage regeneration: a systematic review.Front Cell Dev Biol. 2023 Jul 26;11:1204050. doi: 10.3389/fcell.2023.1204050. eCollection 2023. Front Cell Dev Biol. 2023. PMID: 37564374 Free PMC article. Review.
-
Hypoxia Differentially Affects Chondrogenic Differentiation of Progenitor Cells from Different Origins.Int J Stem Cells. 2023 Aug 30;16(3):304-314. doi: 10.15283/ijsc21242. Epub 2023 Apr 30. Int J Stem Cells. 2023. PMID: 37105555 Free PMC article.
-
Alginate Conjugation Increases Toughness in Auricular Chondrocyte Seeded Collagen Hydrogels.Bioengineering (Basel). 2023 Sep 4;10(9):1037. doi: 10.3390/bioengineering10091037. Bioengineering (Basel). 2023. PMID: 37760139 Free PMC article.
References
-
- Fernandes R. Ear Reconstruction. Local and Regional Flaps Head Neck Reconstruction: A Practical Approach. First. Hoboken, NJ: John Wiley & Sons, Inc., 2015, pp. 170–185
-
- Shieh S., Terada S., and Vacanti J.P. Tissue engineering auricular reconstruction: in vitro and in vivo studies. Biomaterials 25, 1545, 2004 - PubMed
-
- Bichara D.A., O'Sullivan N.-A., Pomerantseva I., Zhao X., Sundback C.A., Vacanti J.P., and Randolph M.A. The tissue-engineered auricle: past, present, and future. Tissue Eng Part B Rev 18, 51, 2012 - PubMed
-
- Angela R., Cao Y.L., Clemente I., Pap S., Vacanti M., Eavey R.D., and Vacanti C.A. Characteristics of cartilage engineered from human pediatric auricular cartilage. Plast Reconstr Surg 103, 1111, 1999 - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

