Caveolin-1 is upregulated in hepatic stellate cells but not sinusoidal endothelial cells after liver injury
- PMID: 26847875
- PMCID: PMC6475201
- DOI: 10.1016/j.tice.2015.12.006
Caveolin-1 is upregulated in hepatic stellate cells but not sinusoidal endothelial cells after liver injury
Abstract
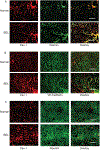
Sinusoidal endothelial cells (SEC) and hepatic stellate cells (HSC) are closely associated specialized vascular cells residing in the hepatic sinusoid. These cells have been shown to play important roles in many different pathophysiologic processes, in particular in liver fibrosis/cirrhosis and portal hypertension. Caveolin-1 functions as a scaffolding protein, and has a variety of functions including in many disease states, such as liver cirrhosis. Although previous studies have shown that in the injured rat liver, caveolin-1 is upregulated, the precise cells in which remains unclear. Therefore, the purpose of this study was to clarify the cell type (or types) in which caveolin-1 is expressed in normal and injured rat liver. We have utilized both detailed immunohistochemical labeling with cell specific markers as well as cell isolation techniques (isolating sinusoidal endothelial cells, HSCs, and hepatocytes) in normal and injured (bile duct ligation) rat liver. We show here that in the normal liver caveolin-1 is expressed predominantly in HSCs and SECs but after liver injury there is upregulation of caveolin-1 in HSCs, but not in SECs. These data have functional implications for the cells in which caveolin-1 is regulated.
Keywords: Immunoblotting; Immunohistochemistry; Nitric oxide; Signaling; eNOS.
Copyright © 2016 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Figures
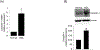

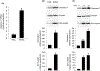
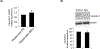
Similar articles
-
Interaction of non‑parenchymal hepatocytes in the process of hepatic fibrosis (Review).Mol Med Rep. 2021 May;23(5):364. doi: 10.3892/mmr.2021.12003. Epub 2021 Mar 24. Mol Med Rep. 2021. PMID: 33760176 Free PMC article. Review.
-
Angiocrine signaling in the hepatic sinusoids in health and disease.Am J Physiol Gastrointest Liver Physiol. 2016 Aug 1;311(2):G246-51. doi: 10.1152/ajpgi.00118.2016. Epub 2016 Jun 10. Am J Physiol Gastrointest Liver Physiol. 2016. PMID: 27288423 Free PMC article. Review.
-
Role of differentiation of liver sinusoidal endothelial cells in progression and regression of hepatic fibrosis in rats.Gastroenterology. 2012 Apr;142(4):918-927.e6. doi: 10.1053/j.gastro.2011.12.017. Epub 2011 Dec 16. Gastroenterology. 2012. PMID: 22178212 Free PMC article.
-
The orphan nuclear receptor COUP-TFII coordinates hypoxia-independent proangiogenic responses in hepatic stellate cells.J Hepatol. 2017 Apr;66(4):754-764. doi: 10.1016/j.jhep.2016.11.003. Epub 2016 Nov 17. J Hepatol. 2017. PMID: 27866920
-
Liver X receptor α is essential for the capillarization of liver sinusoidal endothelial cells in liver injury.Sci Rep. 2016 Feb 18;6:21309. doi: 10.1038/srep21309. Sci Rep. 2016. PMID: 26887957 Free PMC article.
Cited by
-
β-Arrestin2 is a critical component of the GPCR-eNOS signalosome.Proc Natl Acad Sci U S A. 2020 May 26;117(21):11483-11492. doi: 10.1073/pnas.1922608117. Epub 2020 May 13. Proc Natl Acad Sci U S A. 2020. PMID: 32404425 Free PMC article.
-
Caveolin-1 influences mitochondrial plasticity and function in hepatic stellate cell activation.Cell Biol Int. 2022 Nov;46(11):1787-1800. doi: 10.1002/cbin.11876. Epub 2022 Aug 16. Cell Biol Int. 2022. PMID: 35971753 Free PMC article.
-
Caveolin 1 and G-Protein-Coupled Receptor Kinase-2 Coregulate Endothelial Nitric Oxide Synthase Activity in Sinusoidal Endothelial Cells.Am J Pathol. 2017 Apr;187(4):896-907. doi: 10.1016/j.ajpath.2016.11.017. Epub 2017 Feb 3. Am J Pathol. 2017. PMID: 28162981 Free PMC article.
-
Moesin, an Ezrin/Radixin/Moesin Family Member, Regulates Hepatic Fibrosis.Hepatology. 2020 Sep;72(3):1073-1084. doi: 10.1002/hep.31078. Epub 2020 Jul 9. Hepatology. 2020. PMID: 31860744 Free PMC article.
-
Effect of Curcumol on the Fenestrae of Liver Sinusoidal Endothelial Cells Based on NF-κB Signaling Pathway.Evid Based Complement Alternat Med. 2020 May 28;2020:8590638. doi: 10.1155/2020/8590638. eCollection 2020. Evid Based Complement Alternat Med. 2020. PMID: 32595742 Free PMC article.
References
-
- Boyd NL, Park H, Yi H, Boo YC, Sorescu GP, Sykes M, Jo H, 2003. Chronic shear induces caveolae formation and alters ERK and Akt responses in endothelial cells. Am J Physiol Heart Circ Physiol 285, H1113–1122. - PubMed
-
- Connelly L, Madhani M, Hobbs AJ, 2005. Resistance to endotoxic shock in endothelial nitric-oxide synthase (eNOS) knock-out mice: a pro-inflammatory role for eNOS-derived no in vivo. J Biol Chem 280, 10040–10046. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

