Identification of (2S,3S)-β-Methyltryptophan as the Real Biosynthetic Intermediate of Antitumor Agent Streptonigrin
- PMID: 26847951
- PMCID: PMC4742848
- DOI: 10.1038/srep20273
Identification of (2S,3S)-β-Methyltryptophan as the Real Biosynthetic Intermediate of Antitumor Agent Streptonigrin
Abstract
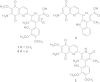
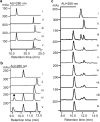
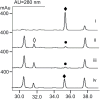
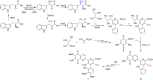
Streptonigrin is a potent antitumor antibiotic, active against a wide range of mammalian tumor cells. It was reported that its biosynthesis relies on (2S,3R)-β-methyltryptophan as an intermediate. In this study, the biosynthesis of (2S,3R)-β-methyltryptophan and its isomer (2S,3S)-β-methyltryptophan by enzymes from the streptonigrin biosynthetic pathway is demonstrated. StnR is a pyridoxal 5'-phosphate (PLP)-dependent aminotransferase that catalyzes a transamination between L-tryptophan and β-methyl indolepyruvate. StnQ1 is an S-adenosylmethionine (SAM)-dependent C-methyltransferase and catalyzes β-methylation of indolepyruvate to generate (R)-β-methyl indolepyruvate. Although StnR exhibited a significant preference for (S)-β-methyl indolepyruvate over the (R)-epimer, StnQ1 and StnR together catalyze (2S,3R)-β-methyltryptophan formation from L-tryptophan. StnK3 is a cupin superfamily protein responsible for conversion of (R)-β-methyl indolepyruvate to its (S)-epimer and enables (2S,3S)-β-methyltryptophan biosynthesis from L-tryptophan when combined with StnQ1 and StnR. Most importantly, (2S,3S)-β-methyltryptophan was established as the biosynthetic intermediate of the streptonigrin pathway by feeding experiments with a knockout mutant, contradicting the previous proposal that stated (2S,3R)-β-methyltryptophan as the intermediate. These data set the stage for the complete elucidation of the streptonigrin biosynthetic pathway, which would unlock the potential of creating new streptonigrin analogues by genetic manipulation of the biosynthetic machinery.
Figures
Similar articles
-
A tryptophan C-methyltransferase involved in streptonigrin biosynthesis in Streptomyces flocculus.Biochem J. 1984 May 15;220(1):309-13. doi: 10.1042/bj2200309. Biochem J. 1984. PMID: 6743267 Free PMC article.
-
Demonstration of a metabolic grid at an early step in the streptonigrin biosynthetic pathway in Streptomyces flocculus.J Antibiot (Tokyo). 1984 Feb;37(2):159-66. doi: 10.7164/antibiotics.37.159. J Antibiot (Tokyo). 1984. PMID: 6706852
-
StnK2 catalysing a Pictet-Spengler reaction involved in the biosynthesis of the antitumor reagent streptonigrin.Org Biomol Chem. 2018 Dec 5;16(47):9124-9128. doi: 10.1039/c8ob02710b. Org Biomol Chem. 2018. PMID: 30483694
-
Chemistry, Biosynthesis and Pharmacology of Streptonigrin: An Old Molecule with Future Prospects for New Drug Design, Development and Therapy.Drug Des Devel Ther. 2023 Apr 8;17:1065-1078. doi: 10.2147/DDDT.S388490. eCollection 2023. Drug Des Devel Ther. 2023. PMID: 37064433 Free PMC article. Review.
-
Alpha-methyltryptophan as a therapeutic agent.Prog Neuropsychopharmacol Biol Psychiatry. 1991;15(6):935-8. doi: 10.1016/0278-5846(91)90020-2. Prog Neuropsychopharmacol Biol Psychiatry. 1991. PMID: 1763198 Review.
Cited by
-
Synthesis of β-Branched Tryptophan Analogues Using an Engineered Subunit of Tryptophan Synthase.J Am Chem Soc. 2016 Jul 13;138(27):8388-91. doi: 10.1021/jacs.6b04836. Epub 2016 Jul 1. J Am Chem Soc. 2016. PMID: 27355405 Free PMC article.
-
Identification of the Antifungal Metabolite Chaetoglobosin P From Discosia rubi Using a Cryptococcus neoformans Inhibition Assay: Insights Into Mode of Action and Biosynthesis.Front Microbiol. 2020 Jul 28;11:1766. doi: 10.3389/fmicb.2020.01766. eCollection 2020. Front Microbiol. 2020. PMID: 32849391 Free PMC article.
-
Xantholipin B produced by the stnR inactivation mutant Streptomyces flocculus CGMCC 4.1223 WJN-1.J Antibiot (Tokyo). 2017 Jan;70(1):90-95. doi: 10.1038/ja.2016.60. Epub 2016 Jun 22. J Antibiot (Tokyo). 2017. PMID: 27328868
-
Tryptophan Synthase Uses an Atypical Mechanism To Achieve Substrate Specificity.Biochemistry. 2016 Dec 27;55(51):7043-7046. doi: 10.1021/acs.biochem.6b01127. Epub 2016 Dec 13. Biochemistry. 2016. PMID: 27935677 Free PMC article.
-
A3 foresight network on natural products.J Ind Microbiol Biotechnol. 2019 Mar;46(3-4):313-317. doi: 10.1007/s10295-018-2111-8. Epub 2018 Nov 24. J Ind Microbiol Biotechnol. 2019. PMID: 30474768
References
-
- Rao K. V. & Cullen W. P. Streptonigrin, an antitumor substance. I. Isolation and characterization. Antibiot. Annu. 7, 950–953 (1959). - PubMed
-
- Rao K. V., Biemann K. & Woodward R. B. The structure of streptonigrin. J. Am. Chem. Soc. 85, 2532–2533 (1963).
-
- Doyle T. W. et al.. Structure determination of lavendamycin-a new antitumor antibiotic from Streptomyces lavendulae. Tetrahedron Lett. 22, 4595–4598 (1981).
-
- Herlt A. J., Rickards R. W. & Wu J.-P. The structure of streptonigrone, and a comment on the biosynthesis of the streptonigrin antibiotics. J. Antibiot. 38, 516–518 (1985). - PubMed
-
- Wang H. et al.. Isolation of streptonigrin and its novel derivative from Micromonospora as inducing agents of p53-dependent cell apoptosis. J. Nat. Prod. 65, 721–724. (2002). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

