Oral versus intravenous iron replacement therapy distinctly alters the gut microbiota and metabolome in patients with IBD
- PMID: 26848182
- PMCID: PMC5531225
- DOI: 10.1136/gutjnl-2015-309940
Oral versus intravenous iron replacement therapy distinctly alters the gut microbiota and metabolome in patients with IBD
Abstract
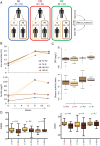
Objective: Iron deficiency is a common complication in patients with IBD and oral iron therapy is suggested to exacerbate IBD symptoms. We performed an open-labelled clinical trial to compare the effects of per oral (PO) versus intravenous (IV) iron replacement therapy (IRT).
Design: The study population included patients with Crohn's disease (CD; N=31), UC (N=22) and control subjects with iron deficiency (non-inflamed, NI=19). After randomisation, participants received iron sulfate (PO) or iron sucrose (IV) over 3 months. Clinical parameters, faecal bacterial communities and metabolomes were assessed before and after intervention.
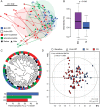
Results: Both PO and IV treatments ameliorated iron deficiency, but higher ferritin levels were observed with IV. Changes in disease activity were independent of iron treatment types. Faecal samples in IBD were characterised by marked interindividual differences, lower phylotype richness and proportions of Clostridiales. Metabolite analysis also showed separation of both UC and CD from control anaemic participants. Major shifts in bacterial diversity occurred in approximately half of all participants after IRT, but patients with CD were most susceptible. Despite individual-specific changes in phylotypes due to IRT, PO treatment was associated with decreased abundances of operational taxonomic units assigned to the species Faecalibacterium prausnitzii, Ruminococcus bromii, Dorea sp. and Collinsella aerofaciens. Clear IV-specific and PO-specific fingerprints were evident at the level of metabolomes, with changes affecting cholesterol-derived host substrates.
Conclusions: Shifts in gut bacterial diversity and composition associated with iron treatment are pronounced in IBD participants. Despite similar clinical outcome, oral administration differentially affects bacterial phylotypes and faecal metabolites compared with IV therapy.
Trial registration number: clinicaltrial.gov (NCT01067547).
Keywords: ANEMIA; IBD CLINICAL; INFLAMMATORY BOWEL DISEASE; INTESTINAL BACTERIA; IRON DEFICIENCY.
Published by the BMJ Publishing Group Limited. For permission to use (where not already granted under a licence) please go to http://www.bmj.com/company/products-services/rights-and-licensing/.
Conflict of interest statement
Figures


Comment in
-
The appropriate dose and cost of iron replacement therapy in patients with IBD.Gut. 2017 Jan;66(1):196-197. doi: 10.1136/gutjnl-2016-311725. Epub 2016 Mar 22. Gut. 2017. PMID: 27006185 No abstract available.
References
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
