Titanium dioxide nanoparticles exacerbate DSS-induced colitis: role of the NLRP3 inflammasome
- PMID: 26848183
- PMCID: PMC5530483
- DOI: 10.1136/gutjnl-2015-310297
Titanium dioxide nanoparticles exacerbate DSS-induced colitis: role of the NLRP3 inflammasome
Abstract
Objective: Western lifestyle and diet are major environmental factors playing a role in the development of IBD. Titanium dioxide (TiO2) nanoparticles are widely used as food additives or in pharmaceutical formulations and are consumed by millions of people on a daily basis. We investigated the effects of TiO2 in the development of colitis and the role of the nucleotide-binding oligomerisation domain receptor, pyrin domain containing (NLRP)3 inflammasome.
Design: Wild-type and NLRP3-deficient mice with dextran sodium sulfate-induced colitis were orally administered with TiO2 nanoparticles. The proinflammatory effects of TiO2 particles in cultured human intestinal epithelial cells (IECs) and macrophages were also studied, as well as the ability of TiO2 crystals to traverse IEC monolayers and accumulate in the blood of patients with IBD using inductively coupled plasma mass spectrometry.
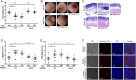
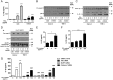
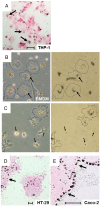
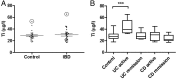
Results: Oral administration of TiO2 nanoparticles worsened acute colitis through a mechanism involving the NLRP3 inflammasome. Importantly, crystals were found to accumulate in spleen of TiO2-administered mice. In vitro, TiO2 particles were taken up by IECs and macrophages and triggered NLRP3-ASC-caspase-1 assembly, caspase-1 cleavage and the release of NLRP3-associated interleukin (IL)-1β and IL-18. TiO2 also induced reactive oxygen species generation and increased epithelial permeability in IEC monolayers. Increased levels of titanium were found in blood of patients with UC having active disease.
Conclusion: These findings indicate that individuals with a defective intestinal barrier function and pre-existing inflammatory condition, such as IBD, might be negatively impacted by the use of TiO2 nanoparticles.
Keywords: IMMUNE RESPONSE; INFLAMMATORY BOWEL DISEASE; INFLAMMATORY MECHANISMS; INTESTINAL BARRIER FUNCTION; REACTIVE OXYGEN SPECIES.
Published by the BMJ Publishing Group Limited. For permission to use (where not already granted under a licence) please go to http://www.bmj.com/company/products-services/rights-and-licensing/.
Conflict of interest statement
Figures


Similar articles
-
Colitis induced in mice with dextran sulfate sodium (DSS) is mediated by the NLRP3 inflammasome.Gut. 2010 Sep;59(9):1192-9. doi: 10.1136/gut.2009.197822. Epub 2010 May 4. Gut. 2010. PMID: 20442201
-
Oral intake of titanium dioxide nanoparticles affect the course and prognosis of ulcerative colitis in mice: involvement of the ROS-TXNIP-NLRP3 inflammasome pathway.Part Fibre Toxicol. 2023 Jun 22;20(1):24. doi: 10.1186/s12989-023-00535-9. Part Fibre Toxicol. 2023. PMID: 37349846 Free PMC article.
-
Galectin-3 Plays an Important Pro-inflammatory Role in the Induction Phase of Acute Colitis by Promoting Activation of NLRP3 Inflammasome and Production of IL-1β in Macrophages.J Crohns Colitis. 2016 May;10(5):593-606. doi: 10.1093/ecco-jcc/jjw013. Epub 2016 Jan 19. J Crohns Colitis. 2016. PMID: 26786981 Free PMC article.
-
Regulation and Sensing of Inflammasomes and Their Impact on Intestinal Health.Int J Mol Sci. 2017 Nov 9;18(11):2379. doi: 10.3390/ijms18112379. Int J Mol Sci. 2017. PMID: 29120406 Free PMC article. Review.
-
NLRP3 Inflammasome and Inflammatory Bowel Disease.Front Immunol. 2019 Feb 28;10:276. doi: 10.3389/fimmu.2019.00276. eCollection 2019. Front Immunol. 2019. PMID: 30873162 Free PMC article. Review.
Cited by
-
Inflammasome-targeting natural compounds in inflammatory bowel disease: Mechanisms and therapeutic potential.Front Immunol. 2022 Aug 24;13:963291. doi: 10.3389/fimmu.2022.963291. eCollection 2022. Front Immunol. 2022. PMID: 36090968 Free PMC article. Review.
-
Crohn's Disease: Is the Cold Chain Hypothesis Still Hot?J Crohns Colitis. 2021 Apr 6;15(4):678-686. doi: 10.1093/ecco-jcc/jjaa192. J Crohns Colitis. 2021. PMID: 32949122 Free PMC article. Review.
-
Food additives can act as triggering factors in celiac disease: Current knowledge based on a critical review of the literature.World J Clin Cases. 2019 Apr 26;7(8):917-927. doi: 10.12998/wjcc.v7.i8.917. World J Clin Cases. 2019. PMID: 31119137 Free PMC article. Review.
-
Jejunal villus absorption and paracellular tight junction permeability are major routes for early intestinal uptake of food-grade TiO2 particles: an in vivo and ex vivo study in mice.Part Fibre Toxicol. 2020 Jun 11;17(1):26. doi: 10.1186/s12989-020-00357-z. Part Fibre Toxicol. 2020. PMID: 32527323 Free PMC article.
-
Hydroxyapatite-coated titanium oxide ameliorates dextran sulphate sodium-induced colitis by attenuating both innate and acquired immune reaction.Prz Gastroenterol. 2023;18(1):76-84. doi: 10.5114/pg.2022.120151. Epub 2022 Oct 6. Prz Gastroenterol. 2023. PMID: 37007758 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
