Crosstalk between adipose-derived stem cells and chondrocytes: when growth factors matter
- PMID: 26848404
- PMCID: PMC4738199
- DOI: 10.1038/boneres.2015.36
Crosstalk between adipose-derived stem cells and chondrocytes: when growth factors matter
Abstract
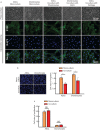
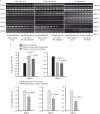
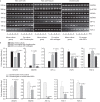
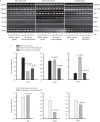
Adipose-derived stem cells (ASCs) and mesenchymal stem cells are promising for tissue repair because of their multilineage differentiation capacity. Our previous data confirmed that the implantation of mixed ASCs and chondrocytes into cartilage defects induced desirable in vivo healing outcomes. However, the paracrine action of ASCs on chondrocytes needs to be further elucidated. In this study, we established a co-culture system to achieve cell-to-cell and cell-to-tissue crosstalk and explored the soluble growth factors in both ASCs and chondrocytes supplemented with 1% fetal bovine serum to mimic the physiological microenvironment. In ASCs, we screened for growth factors by semi-quantitative PCR and quantitative real-time PCR and found that the expression of bone morphogenetic protein 2 (BMP-2), vascular endothelial growth factor B (VEGFB), hypoxia inducible factor-1α (HIF-1α), fibroblast growth factor-2 (FGF-2), and transforming growth factor-β1 significantly increased after co-culture in comparison with mono-culture. In chondrocytes, VEGFA was significantly enhanced after co-culture. Unexpectedly, the expression of collagen II and aggrecan was significantly down-regulated in the co-culture group compared with the mono-culture group. Meanwhile, among all the growth factors screened, we found that the BMP family members BMP-2, BMP-4, and BMP-5 were down-regulated and that VEGFB, HIF-1α, FGF-2, and PDGF were significantly decreased after co-culture. These results suggest that crosstalk between ASCs and chondrocytes is a pathway through the regulated growth factors that might have potential in cartilage repair and regeneration and could be useful for tissue engineering.
Figures

Similar articles
-
Hypoxia enhances angiogenesis in an adipose-derived stromal cell/endothelial cell co-culture 3D gel model.Cell Prolif. 2016 Apr;49(2):236-45. doi: 10.1111/cpr.12244. Epub 2016 Mar 21. Cell Prolif. 2016. PMID: 26997164 Free PMC article.
-
Effects of low oxygen tension on gene profile of soluble growth factors in co-cultured adipose-derived stromal cells and chondrocytes.Cell Prolif. 2016 Jun;49(3):341-51. doi: 10.1111/cpr.12259. Epub 2016 Apr 19. Cell Prolif. 2016. PMID: 27090063 Free PMC article.
-
Gene profile of soluble growth factors involved in angiogenesis, in an adipose-derived stromal cell/endothelial cell co-culture, 3D gel model.Cell Prolif. 2015 Aug;48(4):405-12. doi: 10.1111/cpr.12193. Epub 2015 Jun 1. Cell Prolif. 2015. PMID: 26037311 Free PMC article.
-
Regeneration of articular cartilage by adipose tissue derived mesenchymal stem cells: perspectives from stem cell biology and molecular medicine.J Cell Physiol. 2013 May;228(5):938-44. doi: 10.1002/jcp.24255. J Cell Physiol. 2013. PMID: 23042088 Review.
-
The Regulatory Role of Signaling Crosstalk in Hypertrophy of MSCs and Human Articular Chondrocytes.Int J Mol Sci. 2015 Aug 14;16(8):19225-47. doi: 10.3390/ijms160819225. Int J Mol Sci. 2015. PMID: 26287176 Free PMC article. Review.
Cited by
-
Advanced injectable hydrogels for cartilage tissue engineering.Front Bioeng Biotechnol. 2022 Sep 8;10:954501. doi: 10.3389/fbioe.2022.954501. eCollection 2022. Front Bioeng Biotechnol. 2022. PMID: 36159703 Free PMC article. Review.
-
Recent Advancements in Bone Tissue Engineering: Integrating Smart Scaffold Technologies and Bio-Responsive Systems for Enhanced Regeneration.Int J Mol Sci. 2024 May 30;25(11):6012. doi: 10.3390/ijms25116012. Int J Mol Sci. 2024. PMID: 38892199 Free PMC article. Review.
-
Hypoxia enhances angiogenesis in an adipose-derived stromal cell/endothelial cell co-culture 3D gel model.Cell Prolif. 2016 Apr;49(2):236-45. doi: 10.1111/cpr.12244. Epub 2016 Mar 21. Cell Prolif. 2016. PMID: 26997164 Free PMC article.
-
IGF-1 promotes angiogenesis in endothelial cells/adipose-derived stem cells co-culture system with activation of PI3K/Akt signal pathway.Cell Prolif. 2017 Dec;50(6):e12390. doi: 10.1111/cpr.12390. Epub 2017 Sep 27. Cell Prolif. 2017. PMID: 28960620 Free PMC article.
-
Application of BMP in Bone Tissue Engineering.Front Bioeng Biotechnol. 2022 Mar 31;10:810880. doi: 10.3389/fbioe.2022.810880. eCollection 2022. Front Bioeng Biotechnol. 2022. PMID: 35433652 Free PMC article. Review.
References
-
- Veronesi F, Giavaresi G, Tschon M et al. Clinical use of bone marrow, bone marrow concentrate, and expanded bone marrow mesenchymal stem cells in cartilage disease. Stem Cells Dev 2013; 22: 181–192. - PubMed
-
- Langer R, Vacanti JP. Tissue engineering. Science 1993; 260: 920–926. - PubMed
-
- Kasemkijwattana C, Kesprayura S, Chaipinyo K, Chanlalit C, Chansiri K. Autologous chondrocytes implantation for traumatic cartilage defects of the knee. J Med Assoc Thai 2009; 92: 648–653. - PubMed
-
- Tubo R, Binette F. Culture and identification of autologous human articular chondrocytes for implantation. Methods Mol Med 1999; 18: 205–215. - PubMed
-
- Zheng L, Sun J, Chen X et al. In vivo cartilage engineering with collagen hydrogel and allogenous chondrocytes after diffusion chamber implantation in immunocompetent host. Tissue Eng Part A 2009; 15: 2145–2153. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

