Transport physics and biorheology in the setting of hemostasis and thrombosis
- PMID: 26848552
- PMCID: PMC4870125
- DOI: 10.1111/jth.13280
Transport physics and biorheology in the setting of hemostasis and thrombosis
Abstract
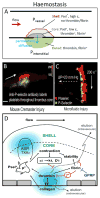
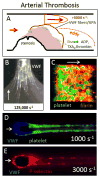
The biophysics of blood flow can dictate the function of molecules and cells in the vasculature with consequent effects on hemostasis, thrombosis, embolism, and fibrinolysis. Flow and transport dynamics are distinct for (i) hemostasis vs. thrombosis and (ii) venous vs. arterial episodes. Intraclot transport changes dramatically the moment hemostasis is achieved or the moment a thrombus becomes fully occlusive. With platelet concentrations that are 50- to 200-fold greater than platelet-rich plasma, clots formed under flow have a different composition and structure compared with blood clotted statically in a tube. The platelet-rich, core/shell architecture is a prominent feature of self-limiting hemostatic clots formed under flow. Importantly, a critical threshold concentration of surface tissue factor is required for fibrin generation under flow. Once initiated by wall-derived tissue factor, thrombin generation and its spatial propagation within a clot can be modulated by γ'-fibrinogen incorporated into fibrin, engageability of activated factor (FIXa)/activated FVIIIa tenase within the clot, platelet-derived polyphosphate, transclot permeation, and reduction of porosity via platelet retraction. Fibrin imparts tremendous strength to a thrombus to resist embolism up to wall shear stresses of 2400 dyne cm(-2) . Extreme flows, as found in severe vessel stenosis or in mechanical assist devices, can cause von Willebrand factor self-association into massive fibers along with shear-induced platelet activation. Pathological von Willebrand factor fibers are A Disintegrin And Metalloprotease with ThromboSpondin-1 domain 13 resistant but are a substrate for fibrin generation due to FXIIa capture. Recently, microfluidic technologies have enhanced the ability to interrogate blood in the context of stenotic flows, acquired von Willebrand disease, hemophilia, traumatic bleeding, and drug action.
Keywords: fibrin; hemodynamics; platelet; shear stress; thrombin; von Willebrand factor.
© 2016 International Society on Thrombosis and Haemostasis.
Conflict of interest statement
L. F. Brass reports grants from The NIH during the conduct of the study as well as personal fees from Merck Pharmaceuticals and Janssen Pharmaceuticals outside the submitted work.
S. L. Diamond has nothing to declare.
Figures
References
-
- Goldsmith HL, Turitto VT. Rheological aspects of thrombosis and haemostasis: basic principles and applications. ICTH-Report--Subcommittee on Rheology of the International Committee on Thrombosis and Haemostasis. Thromb Haemost. 1986;55:415–35. - PubMed
-
- Kefayati S, Holdsworth DW, Poepping TL. Turbulence intensity measurements using particle image velocimetry in diseased carotid artery models: Effect of stenosis severity, plaque eccentricity, and ulceration. J Biomech. 2014;47:253–63. - PubMed
-
- Sivanesan S, How TV, Black RA, Bakran A. Flow patterns in the radiocephalic arteriovenous fistula: an in vitro study. J Biomech. 1999;32:915–25. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

