Intratumoral KIT mutational heterogeneity and recurrent KIT/ PDGFRA mutations in KIT/PDGFRA wild-type gastrointestinal stromal tumors
- PMID: 26848617
- PMCID: PMC5058677
- DOI: 10.18632/oncotarget.7148
Intratumoral KIT mutational heterogeneity and recurrent KIT/ PDGFRA mutations in KIT/PDGFRA wild-type gastrointestinal stromal tumors
Abstract
Objective: Gastrointestinal stromal tumors (GISTs) with no mutations in exons 9, 11, 13, and 17 of the KIT gene and exons 12, and 18 of the PDGFRA gene were defined as KIT/PDGFRA wild-type and they accounted for ~15-20% of GISTs. However, some KIT/PDGFRA wild-type GISTs with KIT mutations in other exons were occasionally reported. We therefore assessed GISTs to understand the whole genomic genotypes of KIT or PDGFRA genes in KIT/PDGFRA wild-type GISTs.
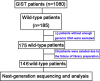
Methods: A cohort of 185 KIT/PDGFRA wild-type GISTs from 1,080 cases was retrospectively assessed. Thirty-nine patients were excluded due to insufficiency of genomic DNA data or failure of library preparation, and 146 patients were analyzed by targeted next-generation sequencing (NGS) followed by validation.
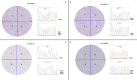
Results: For hot spots in KIT and PDGFRA genes, 23 out of 146 KIT/PDGFRA wild-type cases carried mutations according to NGS; there were 19 KIT mutations and 4 PDGFRA mutations, and these were exclusive. Intratumoral KIT mutational heterogeneity was observed in 4 of 19 samples which potentially triggered mechanisms of polyclonal evolution and metastasis and drug sensitivity. Eleven patients treated with imatinib were evaluable for clinical response, and 2 of 3 patients with KIT mutations achieved partial response (PR), while only 1 of 8 patients without KIT mutations reached PR.
Conclusion: NGS had the potential property to identify partial mutant tumors from a subset of GISTs regarded as KIT/PDGFRA wild-type tumors using Sanger sequencing, and provided a better understanding of KIT/PDGFRA genotypes as well as identified patients eligible for imatinib therapy.
Keywords: KIT/PDGFRA mutation; imatinib; intratumoral heterogeneity; next-generation sequencing; wild-type GISTs.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

Similar articles
-
Gastrointestinal stromal tumors - Summary of mutational status of the primary/secondary KIT/PDGFRA mutations, BRAF mutations and SDH defects.Pathol Res Pract. 2019 Dec;215(12):152708. doi: 10.1016/j.prp.2019.152708. Epub 2019 Oct 29. Pathol Res Pract. 2019. PMID: 31708372
-
Identifying Secondary Mutations in Chinese Patients with Imatinib-Resistant Gastrointestinal Stromal Tumors (GISTs) by Next Generation Sequencing (NGS).Pathol Oncol Res. 2020 Jan;26(1):91-100. doi: 10.1007/s12253-019-00770-6. Epub 2019 Nov 22. Pathol Oncol Res. 2020. PMID: 31758409
-
Concomitant KIT/BRAF and PDGFRA/BRAF mutations are rare events in gastrointestinal stromal tumors.Oncotarget. 2016 May 24;7(21):30109-18. doi: 10.18632/oncotarget.8768. Oncotarget. 2016. PMID: 27097112 Free PMC article.
-
Clinicopathological and molecular features of a large cohort of gastrointestinal stromal tumors (GISTs) and review of the literature: BRAF mutations in KIT/PDGFRA wild-type GISTs are rare events.Hum Pathol. 2017 Apr;62:206-214. doi: 10.1016/j.humpath.2017.01.005. Epub 2017 Feb 1. Hum Pathol. 2017. PMID: 28159677 Review.
-
KIT and PDGFRA mutations in gastrointestinal stromal tumors (GISTs).Semin Diagn Pathol. 2006 May;23(2):91-102. doi: 10.1053/j.semdp.2006.08.006. Semin Diagn Pathol. 2006. PMID: 17193822 Review.
Cited by
-
Intratumoral heterogeneity of c-KIT mutations in a feline splenic mast cell tumor and their functional effects on cell proliferation.Sci Rep. 2022 Sep 22;12(1):15791. doi: 10.1038/s41598-022-19089-5. Sci Rep. 2022. PMID: 36138037 Free PMC article.
-
Comprehensive molecular screening by next generation sequencing reveals a distinctive mutational profile of KIT/PDGFRA genes and novel genomic alterations: results from a 20-year cohort of patients with GIST from north-western Greece.ESMO Open. 2018 Apr 6;3(3):e000335. doi: 10.1136/esmoopen-2018-000335. eCollection 2018. ESMO Open. 2018. PMID: 29636989 Free PMC article.
-
Identification of a GNE homozygous mutation in a Han-Chinese family with GNE myopathy.J Cell Mol Med. 2018 Nov;22(11):5533-5538. doi: 10.1111/jcmm.13827. Epub 2018 Aug 29. J Cell Mol Med. 2018. PMID: 30160005 Free PMC article.
-
Targeted Deep Sequencing Reveals Unrecognized KIT Mutation Coexistent with NF1 Deficiency in GISTs.Cancer Manag Res. 2021 Jan 12;13:297-306. doi: 10.2147/CMAR.S280174. eCollection 2021. Cancer Manag Res. 2021. PMID: 33469372 Free PMC article.
-
Gastrointestinal Stromal Tumours (GISTs) with KRAS Mutation: A Rare but Important Subset of GISTs.Case Rep Gastrointest Med. 2023 Aug 24;2023:4248128. doi: 10.1155/2023/4248128. eCollection 2023. Case Rep Gastrointest Med. 2023. PMID: 37663588 Free PMC article.
References
-
- Joensuu H, Fletcher C, Dimitrijevic S, Silberman S, Roberts P, Demetri G. Management of malignant gastrointestinal stromal tumours. Lancet Oncol. 2002;3:655–64. - PubMed
-
- Wardelmann E, Merkelbach-Bruse S, Pauls K, Thomas N, Schildhaus HU, Heinicke T, Speidel N, Pietsch T, Buettner R, Pink D, Reichardt P, Hohenberger P. Polyclonal evolution of multiple secondary KIT mutations in gastrointestinal stromal tumors under treatment with imatinib mesylate. Clin Cancer Res. 2006;12:1743–9. - PubMed
-
- von Mehren M, Benjamin RS, Bui MM, Casper ES, Conrad EU, 3rd, DeLaney TF, Ganjoo KN, George S, Gonzalez R, Heslin MJ, Kane JM, 3rd, Mayerson J, McGarry SV, et al. Soft tissue sarcoma, version 2. 2012: featured updates to the NCCN guidelines. J Natl Compr Canc Netw. 2012;10:951–60. - PubMed
-
- Oudijk L, Gaal J, Korpershoek E, van Nederveen FH, Kelly L, Schiavon G, Verweij J, Mathijssen RH, den Bakker MA, Oldenburg RA, van Loon RL, O'sullivan MJ, de Krijger RR, et al. SDHA mutations in adult and pediatric wild-type gastrointestinal stromal tumors. Mod Pathol. 2013;26:456–63. - PubMed
-
- Pantaleo MA, Astolfi A, Urbini M, Nannini M, Paterini P, Indio V, Saponara M, Formica S, Ceccarelli C, Casadio R, Rossi G, Bertolini F, Santini D, et al. Analysis of all subunits, SDHA, SDHB, SDHC, SDHD, of the succinate dehydrogenase complex in KIT/PDGFRA wild-type GIST. Eur J Hum Genet. 2014;22:32–9. - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

