Methods to Design and Synthesize Antibody-Drug Conjugates (ADCs)
- PMID: 26848651
- PMCID: PMC4783928
- DOI: 10.3390/ijms17020194
Methods to Design and Synthesize Antibody-Drug Conjugates (ADCs)
Abstract
Antibody-drug conjugates (ADCs) have become a promising targeted therapy strategy that combines the specificity, favorable pharmacokinetics and biodistributions of antibodies with the destructive potential of highly potent drugs. One of the biggest challenges in the development of ADCs is the application of suitable linkers for conjugating drugs to antibodies. Recently, the design and synthesis of linkers are making great progress. In this review, we present the methods that are currently used to synthesize antibody-drug conjugates by using thiols, amines, alcohols, aldehydes and azides.
Keywords: antibody-drug conjugates (ADCs); drugs; linkers; monoclonal antibodies (mAbs); targeted therapy.
Figures




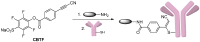


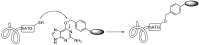
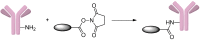

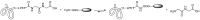
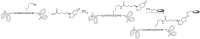
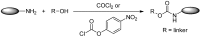

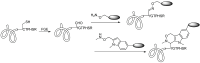




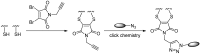
References
-
- Lenoir T. A magic bullet: Research for profit and the growth of knowledge in Germany around 1900. Minerva. 1988;26:66–88. doi: 10.1007/BF01096701. - DOI
-
- Laurent D., Bernhard S. Antibody-drug conjugates: Linking cytotoxic payloads to monoclonal antibodies. Bioconjug. Chem. 2010;21:5–13. - PubMed
-
- Thudium K., Bilic S., Leipold D., Mallet W., Kaur S., Meibohm B., Erickson H., Tibbitts J., Zhao H., Gupta M. American association of pharmaceutical scientists national biotechnology conference short course: Translational challenges in developing antibody-drug conjugates. MAbs. 2013;5:5–12. doi: 10.4161/mabs.22909. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

