Non-genomic activities of retinoic acid receptor alpha control actin cytoskeletal events in human platelets
- PMID: 26848712
- PMCID: PMC5497578
- DOI: 10.1111/jth.13281
Non-genomic activities of retinoic acid receptor alpha control actin cytoskeletal events in human platelets
Abstract
Essentials Platelets employ proteins/signaling pathways traditionally thought reserved for nuclear niche. We determined retinoic-acid-receptor alpha (RARα) expression and function in human platelets. RARα/actin-related protein-2/3 complex (Arp2/3) interact via non-genomic signaling in platelets. RARα regulates Arp2/3-mediated actin cytoskeletal dynamics and platelet spreading.
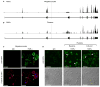
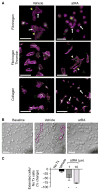
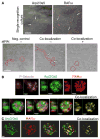
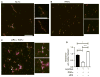
Summary: Background Platelets utilize proteins and pathways classically reserved for the nuclear niche. Methods We determined whether human platelets express retinoic-acid-receptor family members, traditionally thought of as nuclear transcription factors, and deciphered the function of RARα. Results We found that RARα is robustly expressed in human platelets and megakaryocytes and interacts directly with actin-related protein-2/3 complex (Arp2/3) subunit 5 (Arp2/3s5). Arp2/3s5 co-localized with RARα in situ and regulated platelet cytoskeletal processes. The RARα ligand all-trans retinoic acid (atRA) disrupted RARα-Arp2/3 interactions. When isolated human platelets were treated with atRA, rapid cytoskeletal events (e.g. platelet spreading) were inhibited. In addition, when platelets were cultured for 18 h in the presence of atRA, actin-dependent morphological changes (e.g. extended cell body formation) were similarly inhibited. Using in vitro actin branching assays, RARα and Arp2/3-regulated complex actin branch formation was demonstrated. Consistent with inhibition of cytoskeletal processes in platelets, atRA, when added to this branching assay, resulted in dysregulated actin branching. Conclusion Our findings identify a previously unknown mechanism by which RARα regulates Arp2/3-mediated actin cytoskeletal dynamics through a non-genomic signaling pathway. These findings have broad implications in both nucleated and anucleate cells, where actin cytoskeletal events regulate cell morphology, movement and division.
Keywords: actin; actin-related protein 2-3 complex; blood platelets; protein interaction domains and motifs; retinoic acid receptors.
© 2016 International Society on Thrombosis and Haemostasis.
Conflict of interest statement
The authors state that they have no conflict of interest.
Figures


Similar articles
-
Retinoic acid receptor-α regulates synthetic events in human platelets.J Thromb Haemost. 2017 Dec;15(12):2408-2418. doi: 10.1111/jth.13861. Epub 2017 Nov 1. J Thromb Haemost. 2017. PMID: 28981191
-
Arp2/3 complex is required for actin polymerization during platelet shape change.Blood. 2002 Jun 15;99(12):4466-74. doi: 10.1182/blood.v99.12.4466. Blood. 2002. PMID: 12036877 Free PMC article.
-
Novel Zinc Finger Transcription Factor ZFP580 Facilitates All-Trans Retinoic Acid -Induced Vascular Smooth Muscle Cells Differentiation by Rarα-Mediated PI3K/Akt and ERK Signaling.Cell Physiol Biochem. 2018;50(6):2390-2405. doi: 10.1159/000495098. Epub 2018 Nov 13. Cell Physiol Biochem. 2018. PMID: 30423583
-
How WASP-family proteins and the Arp2/3 complex convert intracellular signals into cytoskeletal structures.Curr Opin Cell Biol. 2000 Feb;12(1):91-6. doi: 10.1016/s0955-0674(99)00061-7. Curr Opin Cell Biol. 2000. PMID: 10679362 Review.
-
Cortactin: a multifunctional regulator of cellular invasiveness.Cell Adh Migr. 2011 Mar-Apr;5(2):187-98. doi: 10.4161/cam.5.2.14773. Epub 2011 Mar 1. Cell Adh Migr. 2011. PMID: 21258212 Free PMC article. Review.
Cited by
-
Granzyme A in Human Platelets Regulates the Synthesis of Proinflammatory Cytokines by Monocytes in Aging.J Immunol. 2018 Jan 1;200(1):295-304. doi: 10.4049/jimmunol.1700885. Epub 2017 Nov 22. J Immunol. 2018. PMID: 29167233 Free PMC article.
-
A New Role of NAP1L1 in Megakaryocytes and Human Platelets.Int J Mol Sci. 2022 Nov 24;23(23):14694. doi: 10.3390/ijms232314694. Int J Mol Sci. 2022. PMID: 36499021 Free PMC article.
-
Cobimetinib and trametinib inhibit platelet MEK but do not cause platelet dysfunction.Platelets. 2019;30(6):762-772. doi: 10.1080/09537104.2018.1514107. Epub 2018 Sep 25. Platelets. 2019. PMID: 30252580 Free PMC article.
-
Human platelets display dysregulated sepsis-associated autophagy, induced by altered LC3 protein-protein interaction of the Vici-protein EPG5.Autophagy. 2022 Jul;18(7):1534-1550. doi: 10.1080/15548627.2021.1990669. Epub 2021 Nov 18. Autophagy. 2022. PMID: 34689707 Free PMC article.
-
A ligand-independent fast function of RARα promotes exit from metabolic quiescence upon T cell activation and controls T cell differentiation.Mucosal Immunol. 2021 Jan;14(1):100-112. doi: 10.1038/s41385-020-0311-9. Epub 2020 Jun 9. Mucosal Immunol. 2021. PMID: 32518366 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
- MOP-259952/CIHR/Canada
- MOP-81208/CIHR/Canada
- R01 HL066277/HL/NHLBI NIH HHS/United States
- R03 AG040631/AG/NIA NIH HHS/United States
- U54 HL112311/HL/NHLBI NIH HHS/United States
- K23 HL092161/HL/NHLBI NIH HHS/United States
- R37 HL044525/HL/NHLBI NIH HHS/United States
- K01 GM103806/GM/NIGMS NIH HHS/United States
- R01 AG048022/AG/NIA NIH HHS/United States
- R01 HL075507/HL/NHLBI NIH HHS/United States
- R01 HL126547/HL/NHLBI NIH HHS/United States
- R01 HL044525/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

