Guanylate binding protein-1 mediates EGFRvIII and promotes glioblastoma growth in vivo but not in vitro
- PMID: 26848767
- PMCID: PMC4891076
- DOI: 10.18632/oncotarget.7109
Guanylate binding protein-1 mediates EGFRvIII and promotes glioblastoma growth in vivo but not in vitro
Abstract
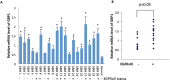
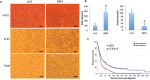
Glioblastoma multiforme (GBM) is the most common and deadly primary brain tumor in adults. Epidermal growth factor receptor (EGFR) is frequently amplified and mutated in GBM. We previously reported that Guanylate binding protein-1 (GBP1) is a novel transcriptional target gene of EGFR and plays a role in GBM invasion. Here we demonstrate that GBP1 can also be induced by EGFRvIII at the transcriptional level through the p38 MAPK/Yin Yang 1 (YY1) signaling pathway. Silencing of GBP1 by RNA interference significantly inhibits EGFRvIII-mediated GBM cell proliferation in vitro and in a mouse model. Overexpression of GBP1 has no obvious effect on glioblastoma cell proliferation in vitro. In contrast, in an orthotopic glioma mouse model GBP1 overexpression significantly promotes glioma growth and reduces survival rate of glioma-bearing mice by increasing cell proliferation and decreasing cell apoptosis in tumor. Clinically, GBP1 expression is elevated in human GBM tumors and positively correlates with EGFRvIII status in GBM specimens, and its expression is inversely correlated with the survival rate of GBM patients. Taken together, these results reveal that GBP1 may serve as a potential therapeutic target for GBMs with EGFRvIII mutation.
Keywords: EGFRvIII; GBP1; glioblastoma; survival; tumor growth.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
-
- Furnari FB, Fenton T, Bachoo RM, Mukasa A, Stommel JM, Stegh A, Hahn WC, Ligon KL, Louis DN, Brennan C, Chin L, DePinho RA, Cavenee WK. Malignant astrocytic glioma: genetics, biology, and paths to treatment. Genes Dev. 2007;21:2683–2710. - PubMed
-
- Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJ, Belanger K, Brandes AA, Marosi C, Bogdahn U, Curschmann J, Janzer RC, Ludwin SK, et al. European Organization for Research and Treatment of Cancer Brain Tumor and Radiotherapy Groups. National Cancer Institute of Canada Clinical Trials Group Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N. Engl. J. Med. 2005;352:987–996. - PubMed
-
- Libermann TA, Nusbaum HR, Razon N, Kris R, Lax I, Soreq H, Whittle N, Waterfield MD, Ullrich A, Schlessinger J. Amplification, enhanced expression and possible rearrangement of EGF receptor gene in primary human brain tumours of glial origin. Nature. 1985;313:144–147. - PubMed
-
- Del Vecchio CA, Li G, Wong AJ. Targeting EGF receptor variant III: tumor-specific peptide vaccination for malignant gliomas. Expert Rev Vaccines. 2012;11:133–44. - PubMed
-
- Verhaak RG, Hoadley KA, Purdom E, Wang V, Qi Y, Wilkerson MD, Miller CR, Ding L, Golub T, Mesirov JP, Alexe G, Lawrence M, O'Kelly M, et al. Cancer Genome Atlas Research Network Integrated genomic analysis identifies clinically relevant subtypes of glioblastoma characterized by abnormalities in PDGFRA, IDH1, EGFR, and NF1. Cancer Cell. 2010;17:98–110. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

