Changes in Rat Brain Tissue Microstructure and Stiffness during the Development of Experimental Obstructive Hydrocephalus
- PMID: 26848844
- PMCID: PMC4743852
- DOI: 10.1371/journal.pone.0148652
Changes in Rat Brain Tissue Microstructure and Stiffness during the Development of Experimental Obstructive Hydrocephalus
Abstract
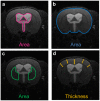
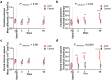
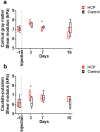
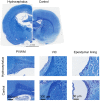
Understanding neural injury in hydrocephalus and how the brain changes during the course of the disease in-vivo remain unclear. This study describes brain deformation, microstructural and mechanical properties changes during obstructive hydrocephalus development in a rat model using multimodal magnetic resonance (MR) imaging. Hydrocephalus was induced in eight Sprague-Dawley rats (4 weeks old) by injecting a kaolin suspension into the cisterna magna. Six sham-injected rats were used as controls. MR imaging (9.4T, Bruker) was performed 1 day before, and at 3, 7 and 16 days post injection. T2-weighted MR images were collected to quantify brain deformation. MR elastography was used to measure brain stiffness, and diffusion tensor imaging (DTI) was conducted to observe brain tissue microstructure. Results showed that the enlargement of the ventricular system was associated with a decrease in the cortical gray matter thickness and caudate-putamen cross-sectional area (P < 0.001, for both), an alteration of the corpus callosum and periventricular white matter microstructure (CC+PVWM) and rearrangement of the cortical gray matter microstructure (P < 0.001, for both), while compression without gross microstructural alteration was evident in the caudate-putamen and ventral internal capsule (P < 0.001, for both). During hydrocephalus development, increased space between the white matter tracts was observed in the CC+PVWM (P < 0.001), while a decrease in space was observed for the ventral internal capsule (P < 0.001). For the cortical gray matter, an increase in extracellular tissue water was significantly associated with a decrease in tissue stiffness (P = 0.001). To conclude, this study characterizes the temporal changes in tissue microstructure, water content and stiffness in different brain regions and their association with ventricular enlargement. In summary, whilst diffusion changes were larger and statistically significant for majority of the brain regions studied, the changes in mechanical properties were modest. Moreover, the effect of ventricular enlargement is not limited to the CC+PVWM and ventral internal capsule, the extent of microstructural changes vary between brain regions, and there is regional and temporal variation in brain tissue stiffness during hydrocephalus development.
Conflict of interest statement
Figures





References
-
- Del Bigio MR. Neuropathological changes caused by hydrocephalus. Acta Neuropathol. 1993;85(6):573–85. - PubMed
-
- Wills KE. Neuropsychological Functioning in Children with Spina-Bifida and or Hydrocephalus. J Clin Child Psychol. 1993;22(2):247–65.
-
- Hanigan WC, Morgan AM, Anderson RJ, Bradle P, Cohen HS, Cusack TJ, et al. Incidence and neurodevelopmental outcome of periventricular hemorrhage and hydrocephalus in a regional population of very low birth weight infants. Neurosurgery. 1991;29(5):701–6. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

