Immunomodulatory and Anti-Inflammatory Activities of Chicken Cathelicidin-2 Derived Peptides
- PMID: 26848845
- PMCID: PMC4743981
- DOI: 10.1371/journal.pone.0147919
Immunomodulatory and Anti-Inflammatory Activities of Chicken Cathelicidin-2 Derived Peptides
Abstract
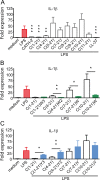
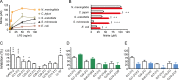
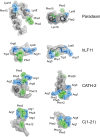
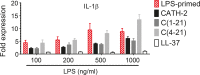
Host Defence Peptides and derived peptides are promising classes of antimicrobial and immunomodulatory lead compounds. For this purpose we examined whether chicken cathelicidin-2 (CATH-2)-derived peptides modulate the function and inflammatory response of avian immune cells. Using a chicken macrophage cell line (HD11) we found that full-length CATH-2 dose-dependently induced transcription of chemokines CXCLi2/IL-8, MCP-3 and CCLi4/RANTES, but not of pro-inflammatory cytokine IL-1β. In addition, CATH-2 efficiently inhibited IL-1β and nitric oxide production by HD11 cells induced by different sources of lipopolysaccharides (LPS). N-terminal truncated CATH-2 derived peptides maintained the capacity to selectively induce chemokine transcription, but despite their high LPS affinity several analogs lacked LPS-neutralizing capacity. Substitution of phenylalanine residues by tryptophan introduced endotoxin neutralization capacity in inactive truncated CATH-2 derived peptides. In contrast, amino acid substitution of phenylalanine by tyrosine abrogated endotoxin neutralization activity of CATH-2 analogs. These findings support a pivotal role for aromatic residues in peptide-mediated endotoxin neutralization by CATH-2 analogs and were shown to be independent of LPS affinity. The capacity to modulate chemokine production and dampen endotoxin-induced pro-inflammatory responses in chicken immune cells implicates that small CATH-2 based peptides could serve as leads for the design of CATH-2 based immunomodulatory anti-infectives.
Conflict of interest statement
Figures



References
-
- Li X, Mehrotra M, Ghimire S, Adewoye L. β-Lactam resistance and β-lactamases in bacteria of animal origin. Vet Mic. 2007;121:197–214. - PubMed
-
- Hancock REW. Mechanisms of action of newer antibiotics for Gram-positive pathogens. Lancet Infect Dis. 2005;5:209–218. - PubMed
-
- Peschel A, Sahl HG. The co-evolution of host cationic antimicrobial peptides and microbial resistance. Nat Rev Microbiol. 2006;4:529–536. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

