Fluorescence In Vivo Hybridization (FIVH) for Detection of Helicobacter pylori Infection in a C57BL/6 Mouse Model
- PMID: 26848853
- PMCID: PMC4743915
- DOI: 10.1371/journal.pone.0148353
Fluorescence In Vivo Hybridization (FIVH) for Detection of Helicobacter pylori Infection in a C57BL/6 Mouse Model
Abstract
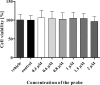
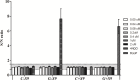
Introduction: In this study, we applied fluorescence in vivo hybridization (FIVH) using locked nucleic acid (LNA) probes targeting the bacterial rRNA gene for in vivo detection of H. pylori infecting the C57BL/6 mouse model. A previously designed Cy3_HP_LNA/2OMe_PS probe, complementary to a sequence of the H. pylori 16S rRNA gene, was used. First, the potential cytotoxicity and genotoxicity of the probe was assessed by commercial assays. Further, the performance of the probe for detecting H. pylori at different pH conditions was tested in vitro, using fluorescence in situ hybridization (FISH). Finally, the efficiency of FIVH to detect H. pylori SS1 strain in C57BL/6 infected mice was evaluated ex vivo in mucus samples, in cryosections and paraffin-embedded sections by epifluorescence and confocal microscopy.
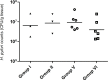
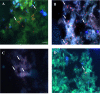
Results: H. pylori SS1 strain infecting C57BL/6 mice was successfully detected by the Cy3_HP_LNA/2OMe_PS probe in the mucus, attached to gastric epithelial cells and colonizing the gastric pits. The specificity of the probe for H. pylori was confirmed by microscopy.
Conclusions: In the future this methodology can be used in combination with a confocal laser endomicroscope for in vivo diagnosis of H. pylori infection using fluorescent LNA probes, which would be helpful to obtain an immediate diagnosis. Our results proved for the first time that FIVH method is applicable inside the body of a higher-order animal.
Conflict of interest statement
Figures




References
-
- Peek RM Jr., Blaser MJ (2002) Helicobacter pylori and gastrointestinal tract adenocarcinomas. Nat Rev Cancer 2: 28–37. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

