Nonthermal ablation in the rat brain using focused ultrasound and an ultrasound contrast agent: long-term effects
- PMID: 26848919
- PMCID: PMC4975676
- DOI: 10.3171/2015.10.JNS151525
Nonthermal ablation in the rat brain using focused ultrasound and an ultrasound contrast agent: long-term effects
Abstract
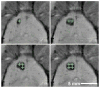
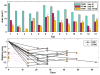
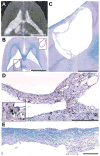
OBJECTIVE Thermal ablation with transcranial MRI-guided focused ultrasound (FUS) is currently under investigation as a less invasive alternative to radiosurgery and resection. A major limitation of the method is that its use is currently restricted to centrally located brain targets. The combination of FUS and a microbubble-based ultrasound contrast agent greatly reduces the ultrasound exposure level needed to ablate brain tissue and could be an effective means to increase the "treatment envelope" for FUS in the brain. This method, however, ablates tissue through a different mechanism: destruction of the microvasculature. It is not known whether nonthermal FUS ablation in substantial volumes of tissue can safely be performed without unexpected effects. The authors investigated this question by ablating volumes in the brains of normal rats. METHODS Overlapping sonications were performed in rats (n = 15) to ablate a volume in 1 hemisphere per animal. The sonications (10-msec bursts at 1 Hz for 60 seconds; peak negative pressure 0.8 MPa) were combined with the ultrasound contrast agent Optison (100 µl/kg). The rats were followed with MRI for 4-9 weeks after FUS, and the brains were examined with histological methods. RESULTS Two weeks after sonication and later, the lesions appeared as cyst-like areas in T2-weighted MR images that were stable over time. Histological examination demonstrated well-defined lesions consisting of a cyst-like cavity that remained lined by astrocytic tissue. Some white matter structures within the sonicated area were partially intact. CONCLUSIONS The results of this study indicate that nonthermal FUS ablation can be used to safely ablate tissue volumes in the brain without unexpected delayed effects. The findings are encouraging for the use of this ablation method in the brain.
Keywords: ETL = echo train length; FOV = field of view; FSE = fast spin echo; FUS = focused ultrasound; H & E = hematoxylin and eosin; LFB = Luxol fast blue; ablation; brain; focused ultrasound.
Conflict of interest statement
The authors report no conflict of interest concerning the materials or methods used in this study or the findings specified in this paper.
Figures






Similar articles
-
Cavitation-enhanced nonthermal ablation in deep brain targets: feasibility in a large animal model.J Neurosurg. 2016 May;124(5):1450-9. doi: 10.3171/2015.4.JNS142862. Epub 2015 Sep 18. J Neurosurg. 2016. PMID: 26381252 Free PMC article.
-
Nonthermal ablation of deep brain targets: A simulation study on a large animal model.Med Phys. 2016 Feb;43(2):870-82. doi: 10.1118/1.4939809. Med Phys. 2016. PMID: 26843248 Free PMC article.
-
Intracranial inertial cavitation threshold and thermal ablation lesion creation using MRI-guided 220-kHz focused ultrasound surgery: preclinical investigation.J Neurosurg. 2015 Jan;122(1):152-61. doi: 10.3171/2014.9.JNS14541. J Neurosurg. 2015. PMID: 25380106
-
Ultrasound Ablation in Neurosurgery: Current Clinical Applications and Future Perspectives.Neurosurgery. 2020 Jul 1;87(1):1-10. doi: 10.1093/neuros/nyz407. Neurosurgery. 2020. PMID: 31745558 Review.
-
Non-invasive transcranial brain ablation with high-intensity focused ultrasound.Front Neurol Neurosci. 2015;36:94-105. doi: 10.1159/000366241. Epub 2014 Dec 22. Front Neurol Neurosci. 2015. PMID: 25531666 Review.
Cited by
-
Intravital imaging and cavitation monitoring of antivascular ultrasound in tumor microvasculature.Theranostics. 2023 Jan 1;13(1):250-266. doi: 10.7150/thno.79186. eCollection 2023. Theranostics. 2023. PMID: 36593952 Free PMC article.
-
For Whom the Bubble Grows: Physical Principles of Bubble Nucleation and Dynamics in Histotripsy Ultrasound Therapy.Ultrasound Med Biol. 2019 May;45(5):1056-1080. doi: 10.1016/j.ultrasmedbio.2018.10.035. Epub 2019 Mar 26. Ultrasound Med Biol. 2019. PMID: 30922619 Free PMC article. Review.
-
Diffusion Tensor Imaging and Chemical Exchange Saturation Transfer MRI Evaluation on the Long-Term Effects of Pulsed Focused Ultrasound and Microbubbles Blood Brain Barrier Opening in the Rat.Front Neurosci. 2020 Aug 25;14:908. doi: 10.3389/fnins.2020.00908. eCollection 2020. Front Neurosci. 2020. PMID: 32982680 Free PMC article.
-
Emerging Therapeutic Strategies for Brain Tumors.Neuromolecular Med. 2022 Mar;24(1):23-34. doi: 10.1007/s12017-021-08681-z. Epub 2021 Aug 18. Neuromolecular Med. 2022. PMID: 34406634 Review.
-
Theranostics in the vasculature: bioeffects of ultrasound and microbubbles to induce vascular shutdown.Theranostics. 2023 Jul 14;13(12):4079-4101. doi: 10.7150/thno.70372. eCollection 2023. Theranostics. 2023. PMID: 37554276 Free PMC article. Review.
References
-
- Aubry JF, Tanter M, Pernot M, Thomas JL, Fink M. Experimental demonstration of noninvasive transskull adaptive focusing based on prior computed tomography scans. J Acoust Soc Am. 2003;113:84–93. - PubMed
-
- Ballantine HT, Jr, Bell E, Manlapaz J. Progress and problems in the neurological applications of focused ultrasound. J Neurosurg. 1960;17:858–876. - PubMed
-
- Bauer R, Martin E, Haegele-Link S, Kaegi G, von Specht M, Werner B. Noninvasive functional neurosurgery using transcranial MR imaging-guided focused ultrasound. Parkinsonism Relat Disord. 2014;20(Suppl 1):S197–S199. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

