Noninvasive brain stimulation treatments for addiction and major depression
- PMID: 26849183
- PMCID: PMC5434820
- DOI: 10.1111/nyas.12985
Noninvasive brain stimulation treatments for addiction and major depression
Abstract
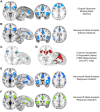
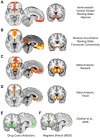
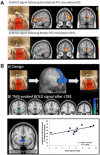
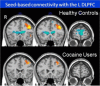
Major depressive disorder (MDD) and substance use disorders (SUDs) are prevalent, disabling, and challenging illnesses for which new treatment options are needed, particularly in comorbid cases. Neuroimaging studies of the functional architecture of the brain suggest common neural substrates underlying MDD and SUDs. Intrinsic brain activity is organized into a set of functional networks, of which two are particularly relevant to psychiatry. The salience network (SN) is crucial for cognitive control and response inhibition, and deficits in SN function are implicated across a wide variety of psychiatric disorders, including MDD and SUDs. The ventromedial network (VMN) corresponds to the classic reward circuit, and pathological VMN activity for drug cues/negative stimuli is seen in SUDs/MDD. Noninvasive brain stimulation (NIBS) techniques, including rTMS and tDCS, have been used to enhance cortico-striatal-thalamic activity through the core SN nodes in the dorsal anterior cingulate cortex, dorsolateral prefrontal cortex, and anterior insula. Improvements in both MDD and SUD symptoms ensue, including in comorbid cases, via enhanced cognitive control. Inhibition of the VMN also appears promising in preclinical studies for quenching the pathological incentive salience underlying SUDs and MDD. Evolving techniques may further enhance the efficacy of NIBS for MDD and SUD cases that are unresponsive to conventional treatments.
Keywords: addiction; depression; fMRI; rTMS; tDCS.
© 2016 The Authors. Annals of the New York Academy of Sciences published by Wiley Periodicals Inc. on behalf of The New York Academy of Sciences.
Figures



Similar articles
-
The Neural Crossroads of Psychiatric Illness: An Emerging Target for Brain Stimulation.Trends Cogn Sci. 2016 Feb;20(2):107-120. doi: 10.1016/j.tics.2015.10.007. Epub 2015 Dec 3. Trends Cogn Sci. 2016. PMID: 26655436 Review.
-
Efficacy of Invasive and Non-Invasive Brain Modulation Interventions for Addiction.Neuropsychol Rev. 2019 Mar;29(1):116-138. doi: 10.1007/s11065-018-9393-5. Epub 2018 Dec 7. Neuropsychol Rev. 2019. PMID: 30536145 Free PMC article. Review.
-
Segregation of salience network predicts treatment response of depression to repetitive transcranial magnetic stimulation.Neuroimage Clin. 2019;22:101719. doi: 10.1016/j.nicl.2019.101719. Epub 2019 Feb 13. Neuroimage Clin. 2019. PMID: 30776777 Free PMC article. Clinical Trial.
-
Cortico-Striatal-Thalamic Loop Circuits of the Salience Network: A Central Pathway in Psychiatric Disease and Treatment.Front Syst Neurosci. 2016 Dec 27;10:104. doi: 10.3389/fnsys.2016.00104. eCollection 2016. Front Syst Neurosci. 2016. PMID: 28082874 Free PMC article. Review.
-
Brain stimulation to treat Internet addiction: A commentary.Addict Behav. 2017 Jan;64:363-364. doi: 10.1016/j.addbeh.2015.11.006. Epub 2015 Nov 26. Addict Behav. 2017. PMID: 26632195 Review.
Cited by
-
The exploration of optimized protocol for repetitive transcranial magnetic stimulation in the treatment of methamphetamine use disorder: A randomized sham-controlled study.EBioMedicine. 2020 Oct;60:103027. doi: 10.1016/j.ebiom.2020.103027. Epub 2020 Sep 25. EBioMedicine. 2020. PMID: 32980696 Free PMC article. Clinical Trial.
-
Metacognitive deficits are associated with lower sensitivity to preference reversals in nicotine dependence.Sci Rep. 2022 Nov 17;12(1):19787. doi: 10.1038/s41598-022-24332-0. Sci Rep. 2022. PMID: 36396945 Free PMC article.
-
Repetitive transcranial magnetic stimulation as a potential treatment approach for cannabis use disorder.Prog Neuropsychopharmacol Biol Psychiatry. 2021 Jul 13;109:110290. doi: 10.1016/j.pnpbp.2021.110290. Epub 2021 Mar 4. Prog Neuropsychopharmacol Biol Psychiatry. 2021. PMID: 33677045 Free PMC article. Review.
-
Changes of frontal cortical subregion volumes in alcohol dependent individuals during early abstinence: associations with treatment outcome.Brain Imaging Behav. 2020 Oct;14(5):1588-1599. doi: 10.1007/s11682-019-00089-5. Brain Imaging Behav. 2020. PMID: 31197582 Free PMC article.
-
Next generation CANCAN focusing for remote stimulation by nanosecond electric pulses.Bioelectrochemistry. 2023 Aug;152:108437. doi: 10.1016/j.bioelechem.2023.108437. Epub 2023 Apr 5. Bioelectrochemistry. 2023. PMID: 37030093 Free PMC article.
References
-
- Whiteford, H.A. , Degenhardt L., Rehm J., et al 2013. Global burden of disease attributable to mental and substance use disorders: findings from the global burden of disease study 2010. Lancet 382: 1575–1586. - PubMed
-
- Rush, A.J. 2007. Limitations in efficacy of antidepressant monotherapy. J. Clin. Psychiatry 68(Suppl.): 8–10. - PubMed
-
- Rush, A.J. , Trivedi M.H., Wisniewski S.R., et al 2006. Acute and longer‐term outcomes in depressed outpatients requiring one or several treatment steps: a STAR*D report. Am. J. Psychiatry 163: 1905–1917. - PubMed
-
- Nemeroff, C.B. 2007. Prevalence and management of treatment‐resistant depression. J. Clin. Psychiatry 68: 17–25. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

