A miR-34a-Numb Feedforward Loop Triggered by Inflammation Regulates Asymmetric Stem Cell Division in Intestine and Colon Cancer
- PMID: 26849305
- PMCID: PMC4751059
- DOI: 10.1016/j.stem.2016.01.006
A miR-34a-Numb Feedforward Loop Triggered by Inflammation Regulates Asymmetric Stem Cell Division in Intestine and Colon Cancer
Abstract
Emerging evidence suggests that microRNAs can initiate asymmetric division, but whether microRNA and protein cell fate determinants coordinate with each other remains unclear. Here, we show that miR-34a directly suppresses Numb in early-stage colon cancer stem cells (CCSCs), forming an incoherent feedforward loop (IFFL) targeting Notch to separate stem and non-stem cell fates robustly. Perturbation of the IFFL leads to a new intermediate cell population with plastic and ambiguous identity. Lgr5+ mouse intestinal/colon stem cells (ISCs) predominantly undergo symmetric division but turn on asymmetric division to curb the number of ISCs when proinflammatory response causes excessive proliferation. Deletion of miR-34a inhibits asymmetric division and exacerbates Lgr5+ ISC proliferation under such stress. Collectively, our data indicate that microRNA and protein cell fate determinants coordinate to enhance robustness of cell fate decision, and they provide a safeguard mechanism against stem cell proliferation induced by inflammation or oncogenic mutation.
Copyright © 2016 Elsevier Inc. All rights reserved.
Figures



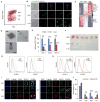
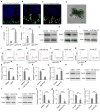
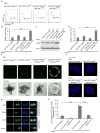
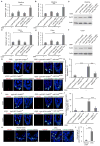
Comment in
-
A role for miR-34 in colon cancer stem cell homeostasis.Stem Cell Investig. 2016 Aug 24;3:42. doi: 10.21037/sci.2016.08.04. eCollection 2016. Stem Cell Investig. 2016. PMID: 27668249 Free PMC article. No abstract available.
Similar articles
-
A microRNA miR-34a-regulated bimodal switch targets Notch in colon cancer stem cells.Cell Stem Cell. 2013 May 2;12(5):602-15. doi: 10.1016/j.stem.2013.03.002. Cell Stem Cell. 2013. PMID: 23642368 Free PMC article.
-
Zeb1 Regulates the Symmetric Division of Mouse Lewis Lung Carcinoma Stem Cells through Numb mediated by miR-31.Int J Biol Sci. 2018 Jul 28;14(11):1399-1410. doi: 10.7150/ijbs.27446. eCollection 2018. Int J Biol Sci. 2018. PMID: 30262992 Free PMC article.
-
The role of NUMB/NUMB isoforms in cancer stem cells.BMB Rep. 2021 Jul;54(7):335-343. doi: 10.5483/BMBRep.2021.54.7.048. BMB Rep. 2021. PMID: 34078527 Free PMC article. Review.
-
TUSC7 suppression of Notch activation through sponging MiR-146 recapitulated the asymmetric cell division in lung adenocarcinoma stem cells.Life Sci. 2019 Sep 1;232:116630. doi: 10.1016/j.lfs.2019.116630. Epub 2019 Jul 4. Life Sci. 2019. PMID: 31279783
-
Numb is involved in the non-random segregation of subcellular vesicles in colorectal cancer stem cells.Cell Cycle. 2016 Oct 17;15(20):2697-703. doi: 10.1080/15384101.2016.1218101. Epub 2016 Aug 11. Cell Cycle. 2016. PMID: 27580100 Free PMC article. Review.
Cited by
-
Farnesoid X receptor via Notch1 directs asymmetric cell division of Sox9+ cells to prevent the development of liver cancer in a mouse model.Stem Cell Res Ther. 2021 Apr 12;12(1):232. doi: 10.1186/s13287-021-02298-6. Stem Cell Res Ther. 2021. PMID: 33845903 Free PMC article.
-
Asymmetric division: An antitumor player?Mol Cell Oncol. 2016 May 19;3(4):e1164279. doi: 10.1080/23723556.2016.1164279. eCollection 2016 Jul. Mol Cell Oncol. 2016. PMID: 27652318 Free PMC article.
-
Endocrine-Disrupting Chemicals and Vitamin D Deficiency in the Pathogenesis of Uterine Fibroids.J Adv Pharm Res. 2021 Spring;5(2):260-275. doi: 10.21608/aprh.2021.66748.1124. J Adv Pharm Res. 2021. PMID: 34746367 Free PMC article.
-
Exact Probability Landscapes of Stochastic Phenotype Switching in Feed-Forward Loops: Phase Diagrams of Multimodality.Front Genet. 2021 Jul 8;12:645640. doi: 10.3389/fgene.2021.645640. eCollection 2021. Front Genet. 2021. PMID: 34306004 Free PMC article.
-
A role for miR-34 in colon cancer stem cell homeostasis.Stem Cell Investig. 2016 Aug 24;3:42. doi: 10.21037/sci.2016.08.04. eCollection 2016. Stem Cell Investig. 2016. PMID: 27668249 Free PMC article. No abstract available.
References
-
- Beckmann J, Scheitza S, Wernet P, Fischer JC, Giebel B. Asymmetric cell division within the human hematopoietic stem and progenitor cell compartment: identification of asymmetrically segregating proteins. Blood. 2007;109:5494–5501. - PubMed
-
- Boon RA, Iekushi K, Lechner S, Seeger T, Fischer A, Heydt S, Kaluza D, Treguer K, Carmona G, Bonauer A, et al. MicroRNA-34a regulates cardiac ageing and function. Nature. 2013;495:107–110. - PubMed
-
- Bouchie A. First microRNA mimic enters clinic. Nature biotechnology. 2013;31:577. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

