Neural Androgen Receptor Deletion Impairs the Temporal Processing of Objects and Hippocampal CA1-Dependent Mechanisms
- PMID: 26849367
- PMCID: PMC4743963
- DOI: 10.1371/journal.pone.0148328
Neural Androgen Receptor Deletion Impairs the Temporal Processing of Objects and Hippocampal CA1-Dependent Mechanisms
Abstract
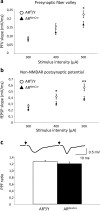
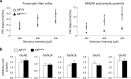
We studied the role of testosterone, mediated by the androgen receptor (AR), in modulating temporal order memory for visual objects. For this purpose, we used male mice lacking AR specifically in the nervous system. Control and mutant males were gonadectomized at adulthood and supplemented with equivalent amounts of testosterone in order to normalize their hormonal levels. We found that neural AR deletion selectively impaired the processing of temporal information for visual objects, without affecting classical object recognition or anxiety-like behavior and circulating corticosterone levels, which remained similar to those in control males. Thus, mutant males were unable to discriminate between the most recently seen object and previously seen objects, whereas their control littermates showed more interest in exploring previously seen objects. Because the hippocampal CA1 area has been associated with temporal memory for visual objects, we investigated whether neural AR deletion altered the functionality of this region. Electrophysiological analysis showed that neural AR deletion affected basal glutamate synaptic transmission and decreased the magnitude of N-methyl-D-aspartate receptor (NMDAR) activation and high-frequency stimulation-induced long-term potentiation. The impairment of NMDAR function was not due to changes in protein levels of receptor. These results provide the first evidence for the modulation of temporal processing of information for visual objects by androgens, via AR activation, possibly through regulation of NMDAR signaling in the CA1 area in male mice.
Conflict of interest statement
Figures




References
-
- Galea LAM, Uban KA, Epp JR, Brummelte S, Barha CK, Wilson WL, et al. Endocrine regulation of cognition and neuroplasticity: our pursuit to unveil the complex interaction between hormones, the brain, and behaviour. Can J Exp Psychol Rev Can Psychol Expérimentale. 2008; 62: 247–260. 10.1037/a0014501 - DOI - PubMed
-
- Howell S, Shalet S. Testosterone deficiency and replacement. Horm Res. 2001; 56 Suppl 1: 86–92. - PubMed
-
- Lamberts SW, van den Beld AW, van der Lely AJ. The endocrinology of aging. Science. 1997; 278: 419–424. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

