Missense Mutations Allow a Sequence-Blind Mutant of SpoIIIE to Successfully Translocate Chromosomes during Sporulation
- PMID: 26849443
- PMCID: PMC4744071
- DOI: 10.1371/journal.pone.0148365
Missense Mutations Allow a Sequence-Blind Mutant of SpoIIIE to Successfully Translocate Chromosomes during Sporulation
Abstract
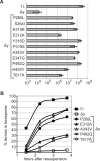
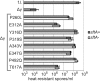
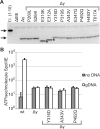
SpoIIIE directionally pumps DNA across membranes during Bacillus subtilis sporulation and vegetative growth. The sequence-reading domain (γ domain) is required for directional DNA transport, and its deletion severely impairs sporulation. We selected suppressors of the spoIIIEΔγ sporulation defect. Unexpectedly, many suppressors were intragenic missense mutants, and some restore sporulation to near-wild-type levels. The mutant proteins are likely not more abundant, faster at translocating DNA, or sequence-sensitive, and rescue does not involve the SpoIIIE homolog SftA. Some mutants behave differently when co-expressed with spoIIIEΔγ, consistent with the idea that some, but not all, variants may form mixed oligomers. In full-length spoIIIE, these mutations do not affect sporulation, and yet the corresponding residues are rarely found in other SpoIIIE/FtsK family members. The suppressors do not rescue chromosome translocation defects during vegetative growth, indicating that the role of the γ domain cannot be fully replaced by these mutations. We present two models consistent with our findings: that the suppressors commit to transport in one arbitrarily-determined direction or delay spore development. It is surprising that missense mutations somehow rescue loss of an entire domain with a complex function, and this raises new questions about the mechanism by which SpoIIIE pumps DNA and the roles SpoIIIE plays in vivo.
Conflict of interest statement
Figures



Similar articles
-
The Bacillus subtilis SftA (YtpS) and SpoIIIE DNA translocases play distinct roles in growing cells to ensure faithful chromosome partitioning.Mol Microbiol. 2009 Nov;74(4):790-809. doi: 10.1111/j.1365-2958.2009.06893.x. Epub 2009 Sep 28. Mol Microbiol. 2009. PMID: 19788545
-
Sequence-directed DNA export guides chromosome translocation during sporulation in Bacillus subtilis.Nat Struct Mol Biol. 2008 May;15(5):485-93. doi: 10.1038/nsmb.1412. Epub 2008 Apr 6. Nat Struct Mol Biol. 2008. PMID: 18391964 Free PMC article.
-
MinCD-dependent regulation of the polarity of SpoIIIE assembly and DNA transfer.EMBO J. 2002 Nov 15;21(22):6267-74. doi: 10.1093/emboj/cdf597. EMBO J. 2002. PMID: 12426398 Free PMC article.
-
FtsK and SpoIIIE, coordinators of chromosome segregation and envelope remodeling in bacteria.Trends Microbiol. 2022 May;30(5):480-494. doi: 10.1016/j.tim.2021.10.002. Epub 2021 Oct 30. Trends Microbiol. 2022. PMID: 34728126 Review.
-
Shaping an Endospore: Architectural Transformations During Bacillus subtilis Sporulation.Annu Rev Microbiol. 2020 Sep 8;74:361-386. doi: 10.1146/annurev-micro-022520-074650. Epub 2020 Jul 13. Annu Rev Microbiol. 2020. PMID: 32660383 Free PMC article. Review.
Cited by
-
Pervasive prophage recombination occurs during evolution of spore-forming Bacilli.ISME J. 2021 May;15(5):1344-1358. doi: 10.1038/s41396-020-00854-1. Epub 2020 Dec 20. ISME J. 2021. PMID: 33343000 Free PMC article.
-
DNA-Membrane Anchor Facilitates Efficient Chromosome Translocation at a Distance in Bacillus subtilis.mBio. 2019 Jun 25;10(3):e01117-19. doi: 10.1128/mBio.01117-19. mBio. 2019. PMID: 31239381 Free PMC article.
-
Single-Molecule Tracking of DNA Translocases in Bacillus subtilis Reveals Strikingly Different Dynamics of SftA, SpoIIIE, and FtsA.Appl Environ Microbiol. 2018 Apr 2;84(8):e02610-17. doi: 10.1128/AEM.02610-17. Print 2018 Apr 15. Appl Environ Microbiol. 2018. PMID: 29439991 Free PMC article.
-
Structural, Metabolic and Evolutionary Comparison of Bacterial Endospore and Exospore Formation.Front Microbiol. 2021 Mar 9;12:630573. doi: 10.3389/fmicb.2021.630573. eCollection 2021. Front Microbiol. 2021. PMID: 33767680 Free PMC article. Review.
-
Xer Site Specific Recombination: Double and Single Recombinase Systems.Front Microbiol. 2017 Mar 20;8:453. doi: 10.3389/fmicb.2017.00453. eCollection 2017. Front Microbiol. 2017. PMID: 28373867 Free PMC article. Review.
References
-
- Wu LJ, Errington J. Bacillus subtilis SpoIIIE protein required for DNA segregation during asymmetric cell division. Science. 1994;264(5158):572–5. Epub 1994/04/22. . - PubMed
-
- Webb CD, Teleman A, Gordon S, Straight A, Belmont A, Lin DC, et al. Bipolar localization of the replication origin regions of chromosomes in vegetative and sporulating cells of B. subtilis. Cell. 1997;88(5):667–74. Epub 1997/03/07. S0092-8674(00)81909-1 [pii]. . - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

