Dynamic DNA methylation controls glutamate receptor trafficking and synaptic scaling
- PMID: 26849493
- PMCID: PMC4836967
- DOI: 10.1111/jnc.13564
Dynamic DNA methylation controls glutamate receptor trafficking and synaptic scaling
Abstract
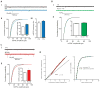
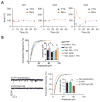
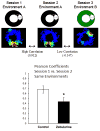
Hebbian plasticity, including long-term potentiation and long-term depression, has long been regarded as important for local circuit refinement in the context of memory formation and stabilization. However, circuit development and stabilization additionally relies on non-Hebbian, homeostatic, forms of plasticity such as synaptic scaling. Synaptic scaling is induced by chronic increases or decreases in neuronal activity. Synaptic scaling is associated with cell-wide adjustments in postsynaptic receptor density, and can occur in a multiplicative manner resulting in preservation of relative synaptic strengths across the entire neuron's population of synapses. Both active DNA methylation and demethylation have been validated as crucial regulators of gene transcription during learning, and synaptic scaling is known to be transcriptionally dependent. However, it has been unclear whether homeostatic forms of plasticity such as synaptic scaling are regulated via epigenetic mechanisms. This review describes exciting recent work that has demonstrated a role for active changes in neuronal DNA methylation and demethylation as a controller of synaptic scaling and glutamate receptor trafficking. These findings bring together three major categories of memory-associated mechanisms that were previously largely considered separately: DNA methylation, homeostatic plasticity, and glutamate receptor trafficking. This review describes exciting recent work that has demonstrated a role for active changes in neuronal DNA methylation and demethylation as a controller of synaptic scaling and glutamate receptor trafficking. These findings bring together three major categories of memory-associated mechanisms that were previously considered separately: glutamate receptor trafficking, DNA methylation, and homeostatic plasticity.
Keywords: AMPA receptor; TET; active demethylation; epigenetic; homeostatic plasticity; memory.
© 2016 International Society for Neurochemistry.
Figures

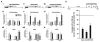

Similar articles
-
Neural plasticity and behavior - sixty years of conceptual advances.J Neurochem. 2016 Oct;139 Suppl 2:179-199. doi: 10.1111/jnc.13580. Epub 2016 Mar 10. J Neurochem. 2016. PMID: 26875778 Review.
-
LTD, LTP, and the sliding threshold for long-term synaptic plasticity.Hippocampus. 1996;6(1):35-42. doi: 10.1002/(SICI)1098-1063(1996)6:1<35::AID-HIPO7>3.0.CO;2-6. Hippocampus. 1996. PMID: 8878740 Review.
-
The AMPA Receptor Code of Synaptic Plasticity.Neuron. 2018 Oct 24;100(2):314-329. doi: 10.1016/j.neuron.2018.10.018. Neuron. 2018. PMID: 30359599 Free PMC article. Review.
-
DNA methylation regulates neuronal glutamatergic synaptic scaling.Sci Signal. 2015 Jun 23;8(382):ra61. doi: 10.1126/scisignal.aab0715. Sci Signal. 2015. PMID: 26106219 Free PMC article.
-
Glutamate receptor trafficking in synaptic plasticity.Sci STKE. 2002 Oct 29;2002(156):re14. doi: 10.1126/stke.2002.156.re14. Sci STKE. 2002. PMID: 12407224 Review.
Cited by
-
DNA Methyltransferase 1 (DNMT1) Shapes Neuronal Activity of Human iPSC-Derived Glutamatergic Cortical Neurons.Int J Mol Sci. 2021 Feb 18;22(4):2034. doi: 10.3390/ijms22042034. Int J Mol Sci. 2021. PMID: 33670788 Free PMC article.
-
Role of DNMTs in the Brain.Adv Exp Med Biol. 2022;1389:363-394. doi: 10.1007/978-3-031-11454-0_15. Adv Exp Med Biol. 2022. PMID: 36350518 Review.
-
Epigenetic Etiology of Intellectual Disability.J Neurosci. 2017 Nov 8;37(45):10773-10782. doi: 10.1523/JNEUROSCI.1840-17.2017. J Neurosci. 2017. PMID: 29118205 Free PMC article. Review.
-
The Interconnected Mechanisms of Oxidative Stress and Neuroinflammation in Epilepsy.Antioxidants (Basel). 2022 Jan 14;11(1):157. doi: 10.3390/antiox11010157. Antioxidants (Basel). 2022. PMID: 35052661 Free PMC article. Review.
-
Memory Synapses Are Defined by Distinct Molecular Complexes: A Proposal.Front Synaptic Neurosci. 2018 Apr 11;10:5. doi: 10.3389/fnsyn.2018.00005. eCollection 2018. Front Synaptic Neurosci. 2018. PMID: 29695960 Free PMC article. Review.
References
-
- Abbott LF, Nelson SB. Synaptic plasticity: taming the beast. Nat Neurosci. 2000;3(Suppl):1178–1183. - PubMed
-
- Alarcon JM, Malleret G, Touzani K, Vronskaya S, Ishii S, Kandel ER, Barco A. Chromatin acetylation, memory, and LTP are impaired in CBP+/- mice: a model for the cognitive deficit in Rubinstein-Taybi syndrome and its amelioration. Neuron. 2004;42:947–959. - PubMed
-
- Baker-Andresen D, Ratnu VS, Bredy TW. Dynamic DNA methylation: a prime candidate for genomic metaplasticity and behavioral adaptation. Trends Neurosci. 2013;36:3–13. - PubMed
-
- Barnes CA, Suster MS, Shen J, McNaughton BL. Multistability of cognitive maps in the hippocampus of old rats. Nature. 1997;388:272–275. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

