gH/gL supercomplexes at early stages of herpesvirus entry
- PMID: 26849495
- PMCID: PMC4970976
- DOI: 10.1016/j.coviro.2016.01.010
gH/gL supercomplexes at early stages of herpesvirus entry
Abstract
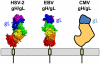
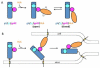
Membrane fusion during herpesvirus entry into host cells is a complex process where multiple glycoproteins interact to relay the triggering signal from a receptor-binding protein to the conserved fusogen gB through the conserved heterodimer gH/gL. Crystal structures of individual glycoproteins are available, yet high-order 'supercomplexes' have been elusive. Recent structures of complexes between gH/gL from human cytomegalovirus or Epstein-Barr virus and the receptor-binding proteins that form at early stages of herpesviral entry highlighted mechanisms that control tropism and revealed dynamic intermediate complexes containing gH/gL that may directly participate in membrane deformation and juxtaposition. Determining how the triggering signal reaches the fusogen gB represents the next frontier in structural biology of herpesvirus entry.
Copyright © 2016 Elsevier B.V. All rights reserved.
Figures


Similar articles
-
Human Cytomegalovirus gH/gL/gO Promotes the Fusion Step of Entry into All Cell Types, whereas gH/gL/UL128-131 Broadens Virus Tropism through a Distinct Mechanism.J Virol. 2015 Sep;89(17):8999-9009. doi: 10.1128/JVI.01325-15. Epub 2015 Jun 17. J Virol. 2015. PMID: 26085146 Free PMC article.
-
Stuck in the middle: structural insights into the role of the gH/gL heterodimer in herpesvirus entry.Curr Opin Virol. 2013 Feb;3(1):13-9. doi: 10.1016/j.coviro.2012.10.005. Epub 2012 Oct 26. Curr Opin Virol. 2013. PMID: 23107819 Free PMC article. Review.
-
Functional Relevance of the N-Terminal Domain of Pseudorabies Virus Envelope Glycoprotein H and Its Interaction with Glycoprotein L.J Virol. 2017 Apr 13;91(9):e00061-17. doi: 10.1128/JVI.00061-17. Print 2017 May 1. J Virol. 2017. PMID: 28228592 Free PMC article.
-
Crystal structure of the Epstein-Barr virus (EBV) glycoprotein H/glycoprotein L (gH/gL) complex.Proc Natl Acad Sci U S A. 2010 Dec 28;107(52):22641-6. doi: 10.1073/pnas.1011806108. Epub 2010 Dec 13. Proc Natl Acad Sci U S A. 2010. PMID: 21149717 Free PMC article.
-
The COMPLEXity in herpesvirus entry.Curr Opin Virol. 2017 Jun;24:97-104. doi: 10.1016/j.coviro.2017.04.006. Epub 2017 May 21. Curr Opin Virol. 2017. PMID: 28538165 Free PMC article. Review.
Cited by
-
Where do we Stand after Decades of Studying Human Cytomegalovirus?Microorganisms. 2020 May 8;8(5):685. doi: 10.3390/microorganisms8050685. Microorganisms. 2020. PMID: 32397070 Free PMC article. Review.
-
A vaccine against cytomegalovirus: how close are we?J Clin Invest. 2025 Jan 2;135(1):e182317. doi: 10.1172/JCI182317. J Clin Invest. 2025. PMID: 39744948 Free PMC article.
-
There Is Always Another Way! Cytomegalovirus' Multifaceted Dissemination Schemes.Viruses. 2018 Jul 20;10(7):383. doi: 10.3390/v10070383. Viruses. 2018. PMID: 30037007 Free PMC article. Review.
-
Different functional states of fusion protein gB revealed on human cytomegalovirus by cryo electron tomography with Volta phase plate.PLoS Pathog. 2018 Dec 3;14(12):e1007452. doi: 10.1371/journal.ppat.1007452. eCollection 2018 Dec. PLoS Pathog. 2018. PMID: 30507948 Free PMC article.
-
Recent Progress in the Vaccine Development Against Epstein-Barr Virus.Viruses. 2025 Jun 30;17(7):936. doi: 10.3390/v17070936. Viruses. 2025. PMID: 40733554 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

