Molecular evolution of troponin I and a role of its N-terminal extension in nematode locomotion
- PMID: 26849746
- PMCID: PMC4846289
- DOI: 10.1002/cm.21281
Molecular evolution of troponin I and a role of its N-terminal extension in nematode locomotion
Abstract
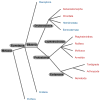
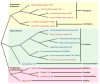
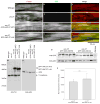
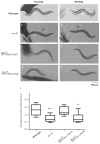
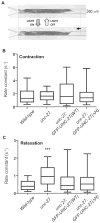
The troponin complex, composed of troponin T (TnT), troponin I (TnI), and troponin C (TnC), is the major calcium-dependent regulator of muscle contraction, which is present widely in both vertebrates and invertebrates. Little is known about evolutionary aspects of troponin in the animal kingdom. Using a combination of data mining and functional analysis of TnI, we report evidence that an N-terminal extension of TnI is present in most of bilaterian animals as a functionally important domain. Troponin components have been reported in species in most of representative bilaterian phyla. Comparison of TnI sequences shows that the core domains are conserved in all examined TnIs, and that N- and C-terminal extensions are variable among isoforms and species. In particular, N-terminal extensions are present in all protostome TnIs and chordate cardiac TnIs but lost in a subset of chordate TnIs including vertebrate skeletal-muscle isoforms. Transgenic rescue experiments in Caenorhabditis elegans striated muscle show that the N-terminal extension of TnI (UNC-27) is required for coordinated worm locomotion but not in sarcomere assembly and single muscle-contractility kinetics. These results suggest that N-terminal extensions of TnIs are retained from a TnI ancestor as a functional domain.
Keywords: actin; contraction; muscle; myosin; troponin.
© 2016 Wiley Periodicals, Inc.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Arad M, Penas-Lado M, Monserrat L, Maron BJ, Sherrid M, Ho CY, Barr S, Karim A, Olson TM, Kamisago M, et al. Gene mutations in apical hypertrophic cardiomyopathy. Circulation. 2005;112:2805–2811. - PubMed
-
- Barbato JC, Huang QQ, Hossain MM, Bond M, Jin JP. Proteolytic N-terminal truncation of cardiac troponin I enhances ventricular diastolic function. J Biol Chem. 2005;280:6602–6609. - PubMed
-
- Blair JE, Hedges SB. Molecular phylogeny and divergence times of deuterostome animals. Mol Biol Evol. 2005;22:2275–2284. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

