Catalyst-Free Synthesis of Borylated Lactones from Esters via Electrophilic Oxyboration
- PMID: 26849770
- PMCID: PMC4768685
- DOI: 10.1021/jacs.5b12989
Catalyst-Free Synthesis of Borylated Lactones from Esters via Electrophilic Oxyboration
Abstract
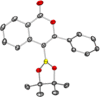
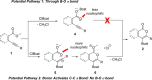
A catalyst-free oxyboration reaction of alkynes is developed. The resulting borylated isocoumarins and 2-pyrones are isolated as boronic acids, pinacolboronate esters, or potassium organotrifluoroborate salts, providing a variety of bench-stable organoboron building blocks for downstream functionalization. This method has functional group compatibility, is scalable, and proceeds with readily available materials: B-chlorocatecholborane and methyl esters. Mechanistic studies indicate that the B-chlorocatecholborane acts as a carbophilic Lewis acid toward the alkyne, providing a mechanistically distinct pathway for oxyboration that avoids B-O σ bond formation and enables this catalyst-free route.
Conflict of interest statement
The authors declare the following competing financial interest(s): U.S. patent no. 9,238,661.
Figures


Similar articles
-
Boron-Heteroatom Addition Reactions via Borylative Heterocyclization: Oxyboration, Aminoboration, and Thioboration.Acc Chem Res. 2017 Oct 17;50(10):2598-2609. doi: 10.1021/acs.accounts.7b00365. Epub 2017 Sep 21. Acc Chem Res. 2017. PMID: 28933550
-
Oxyboration with and without a Catalyst: Borylated Isoxazoles via B-O σ-Bond Addition.Org Lett. 2016 Feb 5;18(3):480-3. doi: 10.1021/acs.orglett.5b03530. Epub 2016 Jan 15. Org Lett. 2016. PMID: 26771389 Free PMC article.
-
Catalyst-Free Formal Thioboration to Synthesize Borylated Benzothiophenes and Dihydrothiophenes.Angew Chem Int Ed Engl. 2016 Nov 7;55(46):14286-14290. doi: 10.1002/anie.201608090. Epub 2016 Oct 13. Angew Chem Int Ed Engl. 2016. PMID: 27735114 Free PMC article.
-
Transition-Metal-Free Synthesis of Borylated Thiophenes via Formal Thioboration.Org Lett. 2018 Nov 2;20(21):6673-6677. doi: 10.1021/acs.orglett.8b02727. Epub 2018 Oct 11. Org Lett. 2018. PMID: 30350646
-
In vitro biosynthesis of polyesters.Adv Biochem Eng Biotechnol. 2001;71:241-62. doi: 10.1007/3-540-40021-4_8. Adv Biochem Eng Biotechnol. 2001. PMID: 11217414 Review.
Cited by
-
B(C6F5)3-catalyzed dehydrogenative cyclization of N-tosylhydrazones and anilines via a Lewis adduct: a combined experimental and computational investigation.Chem Sci. 2019 Jul 5;10(34):7964-7974. doi: 10.1039/c9sc02492a. eCollection 2019 Sep 14. Chem Sci. 2019. PMID: 31853352 Free PMC article.
-
BCl3 -Induced Annulative Oxo- and Thioboration for the Formation of C3-Borylated Benzofurans and Benzothiophenes.Angew Chem Int Ed Engl. 2017 Jan 2;56(1):354-358. doi: 10.1002/anie.201610014. Epub 2016 Nov 29. Angew Chem Int Ed Engl. 2017. PMID: 27897368 Free PMC article.
-
Metal-Free Temperature-Controlled Regiodivergent Borylative Cyclizations of Enynes: BCl3 -Promoted Skeletal Rearrangement.Angew Chem Int Ed Engl. 2022 Jul 11;61(28):e202205651. doi: 10.1002/anie.202205651. Epub 2022 May 24. Angew Chem Int Ed Engl. 2022. PMID: 35510716 Free PMC article.
-
Selective oxymetalation of terminal alkynes via 6-endo cyclization: mechanistic investigation and application to the efficient synthesis of 4-substituted isocoumarins.Chem Sci. 2018 Jun 15;9(28):6041-6052. doi: 10.1039/c8sc01537f. eCollection 2018 Jul 28. Chem Sci. 2018. PMID: 30079217 Free PMC article.
-
Diversification reactions of γ-silyl allenyl esters: selective conversion to all-carbon quaternary centers and γ-allene dicarbinols.Chem Commun (Camb). 2017 May 4;53(37):5125-5127. doi: 10.1039/c7cc01708a. Chem Commun (Camb). 2017. PMID: 28435948 Free PMC article.
References
-
- Brown H. C. Tetrahedron 1961, 12, 117.10.1016/0040-4020(61)80107-5. - DOI
- Miyaura N.Hydroboration, Diboration, and Stannylboration. In Catalytic Heterofunctionalization; Togni A., Grützmacher H., Eds.; Wiley-VCH: Weinheim, 2001; pp 1–46.
- Barbeyron R.; Benedetti E.; Cossy J.; Vasseur J.; Arseniyadis S.; Smietana M. Tetrahedron 2014, 70, 8431.10.1016/j.tet.2014.06.026. - DOI
- Sakae R.; Hirano K.; Miura M. J. Am. Chem. Soc. 2015, 137, 6460.10.1021/jacs.5b02775. - DOI - PubMed
- Hara S.; Dojo H.; Takinami S.; Suzuki A. Tetrahedron Lett. 1983, 24, 731.10.1016/S0040-4039(00)81511-7. - DOI
- Cade I. A.; Ingleson M. J. Chem. - Eur. J. 2014, 20, 12874.10.1002/chem.201403614. - DOI - PMC - PubMed
-
- Sanderson R. T.Polar Covalence; Academic Press: San Diego, CA, 1983.
-
- Roy S.; Roy S.; Neuenswander B.; Hill D.; Larock R. C. J. Comb. Chem. 2009, 11, 1128.10.1021/cc9001197. - DOI - PMC - PubMed
- Pal S.; Chatare V.; Pal M. Curr. Org. Chem. 2011, 15, 782.10.2174/138527211794518970. - DOI
- Yao T.; Larock R. C. J. Org. Chem. 2003, 68, 5936.10.1021/jo034308v. - DOI - PubMed
- Oliver M. A.; Gandour R. D. J. Org. Chem. 1984, 49, 558.10.1021/jo00177a038. - DOI
- Yao T.; Larock R. C. Tetrahedron Lett. 2002, 43, 7401.10.1016/S0040-4039(02)01731-8. - DOI
- Rossi R.; Carpita A.; Bellina F.; Stabile P.; Mannina L. Tetrahedron 2003, 59, 2067.10.1016/S0040-4020(03)00212-6. - DOI
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

