Exploratory Biomarker Study of the Triple Reuptake Inhibitor SEP-432 Compared to the Dual Reuptake Inhibitor Duloxetine in Healthy Normal Subjects
- PMID: 26849844
- PMCID: PMC6492792
- DOI: 10.1111/cns.12513
Exploratory Biomarker Study of the Triple Reuptake Inhibitor SEP-432 Compared to the Dual Reuptake Inhibitor Duloxetine in Healthy Normal Subjects
Abstract
Introduction: SEP-432 is a triple monoamine reuptake inhibitor of norepinephrine (NE), serotonin (5-HT), and dopamine (DA), based on in vitro binding studies. We sought evidence that SEP-432 engages these monoamine systems by measuring concentrations of monoamines and/or their main metabolites in cerebrospinal fluid (CSF) and plasma and comparing results to duloxetine, a dual reuptake inhibitor of NE and 5-HT.
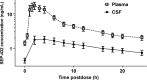
Methods: Eighteen healthy normal subjects received either SEP-432 (300 mg/day), duloxetine (60 mg/day), or placebo for 14 days in-clinic (double blind) with CSF and plasma collections at baseline (single lumbar puncture) and Day 14 (24-h CSF and plasma collection). Concentrations of monoamines and their metabolites, as well as pharmacokinetic concentrations of SEP-432 and metabolite, were quantified by liquid chromatography-tandem mass spectrometry.
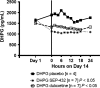
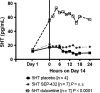
Results: Compared to placebo in the Day 14 area under the curve 24-h (AUC0-24 h ) analysis, SEP-432 significantly (P < 0.05) decreased the NE metabolite dihydroxyphenylglycol (DHPG) in CSF and plasma, decreased 5-HT in plasma, and did not affect DA metabolites, while duloxetine had significant effects on DHPG and 5-HT. Time-matched baseline to Day 14 biomarker comparisons confirmed these findings.
Conclusion: CSF monoamine biomarkers confirmed central NET activity for SEP-432 and duloxetine's dual reuptake inhibition.
Keywords: Biomarkers; Cerebrospinal fluid; Duloxetine; Monoamine reuptake inhibitor; SEP-432.
© 2016 John Wiley & Sons Ltd.
Conflict of interest statement
This study was sponsored by Sunovion Pharmaceuticals Inc.
Drs. Hardy, Versavel, Kharidia, Grinnell, and Chen are (or were at the time the studies described were conducted) employees of Sunovion Pharmaceuticals Inc. Drs. Sramek, Bieck, Zamora, Cutler, and Mr. Sullivan and Mr. Ding are employees of Worldwide Clinical Trials, Inc.
Drs. Sramek, Bieck, Zamora, Cutler, and Mr. Sullivan and Mr. Ding, in the past 2 years, have not received any direct research support and/or consulting fees.
Figures
References
-
- Praag HM. The Harold E. Himwich Memorial Lecture. Significance of biochemical parameters in the diagnosis, treatment, and prevention of depressive disorders. Biol Psychiatry 1977;12:101–131. - PubMed
-
- Aberg‐Wistedt A, Jostell KG, Ross SB, Westerlund D. Effects of zimelidine and desipramine on serotonin and noradrenaline uptake mechanisms in relation to plasma concentrations and to therapeutic effects during treatment of depression. Psychopharmacology 1981;74:297–305. - PubMed
-
- Little JT, Ketter TA, Mathe AA, Frye MA, Luckenbaugh D, Post RM. Venlafaxine but not bupropion decreases cerebrospinal fluid 5‐hydroxyindoleacetic acid in unipolar depression. Biol Psychiatry 1999;45:285–289. - PubMed
-
- Sheline Y, Bardgett ME, Csernansky JG. Correlated reductions in cerebrospinal fluid 5‐HIAA and MHPG concentrations after treatment with selective serotonin reuptake inhibitors. J Clin Psychopharmacol 1997;17:11–14. - PubMed
-
- Golden RN, Rudorfer MV, Sherer MA, Linnoila M, Potter WZ. Bupropion in depression. I. Biochemical effects and clinical response. Arch Gen Psychiatry 1988;45:139–143. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

