Repression of harmful meiotic recombination in centromeric regions
- PMID: 26849908
- PMCID: PMC4867242
- DOI: 10.1016/j.semcdb.2016.01.042
Repression of harmful meiotic recombination in centromeric regions
Abstract
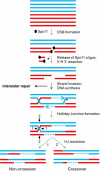
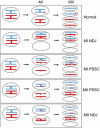
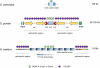
During the first division of meiosis, segregation of homologous chromosomes reduces the chromosome number by half. In most species, sister chromatid cohesion and reciprocal recombination (crossing-over) between homologous chromosomes are essential to provide tension to signal proper chromosome segregation during the first meiotic division. Crossovers are not distributed uniformly throughout the genome and are repressed at and near the centromeres. Rare crossovers that occur too near or in the centromere interfere with proper segregation and can give rise to aneuploid progeny, which can be severely defective or inviable. We review here how crossing-over occurs and how it is prevented in and around the centromeres. Molecular mechanisms of centromeric repression are only now being elucidated. However, rapid advances in understanding crossing-over, chromosome structure, and centromere functions promise to explain how potentially deleterious crossovers are avoided in certain chromosomal regions while allowing beneficial crossovers in others.
Keywords: Aneuploidy; Centromeres; Chromosome segregation; Crossing-over; Homologous recombination; Meiosis.
Copyright © 2016 Elsevier Ltd. All rights reserved.
Figures
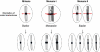
References
-
- Przewloka MR, Glover DM. The kinetochore and the centromere: a working long distance relationship. Annu Rev Genet. 2009;43:439–465. - PubMed
-
- Keeney S. Spo11 and the formation of DNA double-strand breaks in meiosis. In: Egel R, Lankenau D-H, editors. Recombination and meiosis: crossing-over and disjunction. Springer-Verlag; Berlin: 2007. pp. 81–123.
-
- Smith KN, Nicolas A. Recombination at work for meiosis. Current Opinion in Genetics and Development. 1998;8:200–211. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

