Persister formation in Escherichia coli can be inhibited by treatment with nitric oxide
- PMID: 26849946
- PMCID: PMC4898466
- DOI: 10.1016/j.freeradbiomed.2016.02.003
Persister formation in Escherichia coli can be inhibited by treatment with nitric oxide
Abstract
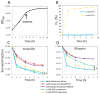
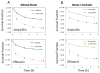
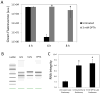
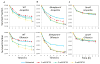
Bacterial persisters are phenotypic variants that survive extraordinary concentrations of antibiotics, and are thought to underlie the propensity of biofilm infections to relapse. Unfortunately many aspects of persister physiology remain ill-defined, which prevents progress toward eradicating the phenotype. Recently, we identified respiration within non-growing Escherichia coli populations as a potential target for the elimination type I persisters, which are those that arise from passage through stationary phase. Here we discovered that nitric oxide (NO) treatment at the onset of stationary phase significantly reduced type I persister formation through its ability to inhibit respiration. NO decreased protein and RNA degradation in stationary phase cells, and produced populations that were more fit for protein synthesis and growth resumption upon introduction into fresh media than untreated controls. Overall, this data shows that NO, which is a therapeutically-relevant compound, has the potential to decrease the incidence of recurrent infections from persisters.
Copyright © 2016 Elsevier Inc. All rights reserved.
Figures
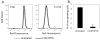
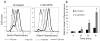
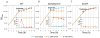
Similar articles
-
Investigating the Effects of Osmolytes and Environmental pH on Bacterial Persisters.Antimicrob Agents Chemother. 2020 Apr 21;64(5):e02393-19. doi: 10.1128/AAC.02393-19. Print 2020 Apr 21. Antimicrob Agents Chemother. 2020. PMID: 32094133 Free PMC article.
-
Stationary phase persister formation in Escherichia coli can be suppressed by piperacillin and PBP3 inhibition.BMC Microbiol. 2019 Jun 24;19(1):140. doi: 10.1186/s12866-019-1506-7. BMC Microbiol. 2019. PMID: 31234796 Free PMC article.
-
Inhibition of stationary phase respiration impairs persister formation in E. coli.Nat Commun. 2015 Aug 6;6:7983. doi: 10.1038/ncomms8983. Nat Commun. 2015. PMID: 26246187 Free PMC article.
-
Multidrug tolerance of biofilms and persister cells.Curr Top Microbiol Immunol. 2008;322:107-31. doi: 10.1007/978-3-540-75418-3_6. Curr Top Microbiol Immunol. 2008. PMID: 18453274 Review.
-
Persister cells and the riddle of biofilm survival.Biochemistry (Mosc). 2005 Feb;70(2):267-74. doi: 10.1007/s10541-005-0111-6. Biochemistry (Mosc). 2005. PMID: 15807669 Review.
Cited by
-
Sublethal Paraquat Confers Multidrug Tolerance in Pseudomonas aeruginosa by Inducing Superoxide Dismutase Activity and Lowering Envelope Permeability.Front Microbiol. 2020 Sep 25;11:576708. doi: 10.3389/fmicb.2020.576708. eCollection 2020. Front Microbiol. 2020. PMID: 33101252 Free PMC article.
-
Monitoring Persister Resuscitation with Flow Cytometry.Methods Mol Biol. 2021;2357:209-222. doi: 10.1007/978-1-0716-1621-5_14. Methods Mol Biol. 2021. PMID: 34590261
-
Bactericidal activity of alpha-bromocinnamaldehyde against persisters in Escherichia coli.PLoS One. 2017 Jul 27;12(7):e0182122. doi: 10.1371/journal.pone.0182122. eCollection 2017. PLoS One. 2017. PMID: 28750057 Free PMC article.
-
High-Throughput Screening of a Promoter Library Reveals New Persister Mechanisms in Escherichia Coli.Microbiol Spectr. 2022 Feb 23;10(1):e0225321. doi: 10.1128/spectrum.02253-21. Epub 2022 Feb 23. Microbiol Spectr. 2022. PMID: 35196813 Free PMC article.
-
Investigating the Effects of Osmolytes and Environmental pH on Bacterial Persisters.Antimicrob Agents Chemother. 2020 Apr 21;64(5):e02393-19. doi: 10.1128/AAC.02393-19. Print 2020 Apr 21. Antimicrob Agents Chemother. 2020. PMID: 32094133 Free PMC article.
References
-
- Amato SM, Orman MA, Brynildsen MP. Metabolic Control of Persister Formation in Escherichia coli. Molecular Cell. 2013;50:475–487. - PubMed
-
- Andrews JM. Determination of minimum inhibitory concentrations. Journal of Antimicrobial Chemotherapy. 2001;48:5–16. - PubMed
-
- Balaban NQ, Gerdes K, Lewis K, McKinney JD. A problem of persistence: still more questions than answers? Nature Reviews Microbiology. 2013;11:587–591. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

