A Glycosyltransferase from Nicotiana alata Pollen Mediates Synthesis of a Linear (1,5)-α-L-Arabinan When Expressed in Arabidopsis
- PMID: 26850276
- PMCID: PMC4825119
- DOI: 10.1104/pp.15.02005
A Glycosyltransferase from Nicotiana alata Pollen Mediates Synthesis of a Linear (1,5)-α-L-Arabinan When Expressed in Arabidopsis
Abstract
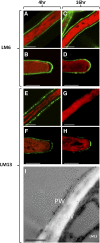
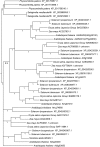
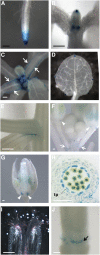
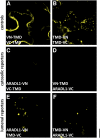
The walls of Nicotiana alata pollen tubes contain a linear arabinan composed of (1,5)-α-linked arabinofuranose residues. Although generally found as a side chain on the backbone of the pectic polysaccharide rhamnogalacturonan I, the arabinan in N. alata pollen tubes is considered free, as there is no detectable rhamnogalacturonan I in these walls. Carbohydrate-specific antibodies detected arabinan epitopes at the tip and along the shank of N. alata pollen tubes that are predominantly part of the primary layer of the bilayered wall. A sequence related to ARABINAN DEFICIENT1 (AtARAD1), a presumed arabinan arabinosyltransferase from Arabidopsis (Arabidopsis thaliana), was identified by searching an N alata pollen transcriptome. Transcripts for this ARAD1-like sequence, which we have named N. alata ARABINAN DEFICIENT-LIKE1 (NaARADL1), accumulate in various tissues, most abundantly in the pollen grain and tube, and encode a protein that is a type II membrane protein with its catalytic carboxyl terminus located in the Golgi lumen. The NaARADL1 protein can form homodimers when transiently expressed in Nicotiana benthamiana leaves and heterodimers when coexpressed with AtARAD1 The expression of NaARADL1 in Arabidopsis led to plants with more arabinan in their walls and that also exuded a guttation fluid rich in arabinan. Chemical and enzymatic characterization of the guttation fluid showed that a soluble, linear α-(1,5)-arabinan was the most abundant polymer present. These results are consistent with NaARADL1 having an arabinan (1,5)-α-arabinosyltransferase activity.
© 2016 American Society of Plant Biologists. All Rights Reserved.
Figures


Similar articles
-
A DUF-246 family glycosyltransferase-like gene affects male fertility and the biosynthesis of pectic arabinogalactans.BMC Plant Biol. 2016 Apr 18;16:90. doi: 10.1186/s12870-016-0780-x. BMC Plant Biol. 2016. PMID: 27091363 Free PMC article.
-
ARAD proteins associated with pectic Arabinan biosynthesis form complexes when transiently overexpressed in planta.Planta. 2012 Jul;236(1):115-28. doi: 10.1007/s00425-012-1592-3. Epub 2012 Jan 21. Planta. 2012. PMID: 22270560
-
ARABINAN DEFICIENT 1 is a putative arabinosyltransferase involved in biosynthesis of pectic arabinan in Arabidopsis.Plant Physiol. 2006 Jan;140(1):49-58. doi: 10.1104/pp.105.072744. Epub 2005 Dec 23. Plant Physiol. 2006. PMID: 16377743 Free PMC article.
-
Metabolism of L-arabinose in plants.J Plant Res. 2016 Sep;129(5):781-792. doi: 10.1007/s10265-016-0834-z. Epub 2016 May 24. J Plant Res. 2016. PMID: 27220955 Free PMC article. Review.
-
Plant glycosyltransferases.Curr Opin Plant Biol. 2001 Jun;4(3):219-24. doi: 10.1016/s1369-5266(00)00164-3. Curr Opin Plant Biol. 2001. PMID: 11312132 Review.
Cited by
-
Tip Growth Defective1 interacts with the cellulose synthase complex to regulate cellulose synthesis in Arabidopsis thaliana.PLoS One. 2024 Feb 15;19(2):e0292149. doi: 10.1371/journal.pone.0292149. eCollection 2024. PLoS One. 2024. PMID: 38358988 Free PMC article.
-
Multiprotein Complexes of Plant Glycosyltransferases Involved in Their Function and Trafficking.Plants (Basel). 2025 Jan 24;14(3):350. doi: 10.3390/plants14030350. Plants (Basel). 2025. PMID: 39942912 Free PMC article. Review.
-
Colocalising proteins and polysaccharides in plants for cell wall and trafficking studies.Front Plant Sci. 2024 Sep 11;15:1440885. doi: 10.3389/fpls.2024.1440885. eCollection 2024. Front Plant Sci. 2024. PMID: 39328792 Free PMC article.
-
Gamete expression of TALE class HD genes activates the diploid sporophyte program in Marchantia polymorpha.Elife. 2021 Sep 17;10:e57088. doi: 10.7554/eLife.57088. Elife. 2021. PMID: 34533136 Free PMC article.
-
Evolution of Cell Wall Polymers in Tip-Growing Land Plant Gametophytes: Composition, Distribution, Functional Aspects and Their Remodeling.Front Plant Sci. 2019 Apr 18;10:441. doi: 10.3389/fpls.2019.00441. eCollection 2019. Front Plant Sci. 2019. PMID: 31057570 Free PMC article. Review.
References
-
- Anisimova M, Gascuel O (2006) Approximate likelihood-ratio test for branches: a fast, accurate, and powerful alternative. Syst Biol 55: 539–552 - PubMed
-
- Atmodjo MA, Hao Z, Mohnen D (2013) Evolving views of pectin biosynthesis. Annu Rev Plant Biol 64: 747–779 - PubMed
-
- Atmodjo MA, Sakuragi Y, Zhu X, Burrell AJ, Mohanty SS, Atwood JA III, Orlando R, Scheller HV, Mohnen D (2011) Galacturonosyltransferase (GAUT)1 and GAUT7 are the core of a plant cell wall pectin biosynthetic homogalacturonan:galacturonosyltransferase complex. Proc Natl Acad Sci USA 108: 20225–20230 - PMC - PubMed
-
- Beveridge T, Wrolstad RE (1997) Haze and cloud in apple juices. Crit Rev Food Sci Nutr 37: 75–91 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

