Acute blockade of the Caenorhabditis elegans dopamine transporter DAT-1 by the mammalian norepinephrine transporter inhibitor nisoxetine reveals the influence of genetic modifications of dopamine signaling in vivo
- PMID: 26850478
- PMCID: PMC4969213
- DOI: 10.1016/j.neuint.2016.01.008
Acute blockade of the Caenorhabditis elegans dopamine transporter DAT-1 by the mammalian norepinephrine transporter inhibitor nisoxetine reveals the influence of genetic modifications of dopamine signaling in vivo
Abstract
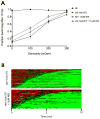
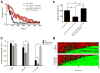
Modulation of neurotransmission by the catecholamine dopamine (DA) is conserved across phylogeny. In the nematode Caenorhabditis elegans, excess DA signaling triggers Swimming-Induced Paralysis (Swip), a phenotype first described in animals with loss of function mutations in the presynaptic DA transporter (dat-1). Swip has proven to be a phenotype suitable for the identification of novel dat-1 mutations as well as the identification of novel genes that impact DA signaling. Pharmacological manipulations can also induce Swip, though the reagents employed to date lack specificity and potency, limiting their use in evaluation of dat-1 expression and function. Our lab previously established the mammalian norepinephrine transporter (NET) inhibitor nisoxetine to be a potent antagonist of DA uptake conferred by DAT-1 following heterologous expression. Here we demonstrate the ability of low (μM) concentrations of nisoxetine to trigger Swip within minutes of incubation, with paralysis dependent on DA release and signaling, and non-additive with Swip triggered by dat-1 deletion. Using nisoxetine in combination with genetic mutations that impact DA release, we further demonstrate the utility of the drug for demonstrating contributions of presynaptic DA receptors and ion channels to Swip. Together, these findings reveal nisoxetine as a powerful reagent for monitoring multiple dimensions of DA signaling in vivo, thus providing a new resource that can be used to evaluate contributions of dat-1 and other genes linked to DA signaling without the potential for compensations that attend constitutive genetic mutations.
Keywords: Caenorhabditiselegans; Dopamine; Nematode; Nisoxetine; Transporter.
Copyright © 2016 Elsevier Ltd. All rights reserved.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

